
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 21P
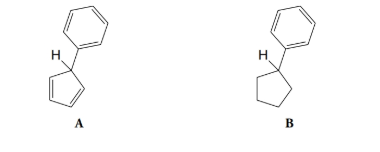
Which of the hydrogen atoms shown below is more acidic? Explain your answer.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In the image below, correctly name the glassware # _P ( Blank 1) and T ( Blank 2).
景
A
W
Blank # 1
Blank #2
1000
+19
E
E
D
0
0-0
G
H
A
A
K
Π
12
R
M
N
S
0-0-
Feedback: Your answer is incorrect.
Predict the major products of the following organic reaction:
CN
Δ
+
A ?
NC
Some important notes:
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
• Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers.
esc
Check
80
MH
F1
F2
F3
F4
F5
50
@
# C
%
95
€
Save For Later
Sub
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C
A
DII
F6
F7
F8
7
*
8
Λ
&
6
F9
F10
9
0
4
Incorrect
Feedback: Your answer is incorrect.
Predict the major products of the following organic reaction:
ཤིགས་བྱ རྩ་ཅད་ཀྱིས་༢༩
+
Some important notes:
A
^ ?
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
• Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers.
E
Check
0
لا
Save For La
©2025 McGraw Hill LLC. All Rights Reserved. Terms of
All
F9
A
Chapter 14 Solutions
Organic Chemistry
Ch. 14 - PRACTICE PROBLEM 14.1 Provide a name for each of...Ch. 14 - Prob. 2PPCh. 14 - Prob. 3PPCh. 14 - Practice Problem 14.4 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.5 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.6 1,3,5-Cycloheptatriene is...Ch. 14 - Prob. 7PPCh. 14 - Prob. 8PPCh. 14 - Practice Problem 14.9 In 1967 R. Breslow (of...Ch. 14 - Prob. 10PP
Ch. 14 - Practice Problem 14.11 In addition to a signal...Ch. 14 - PRACTICE PROBLEM 14.12
Azulene has an appreciable...Ch. 14 - Practice Problem 14.13 (a) The -Sh group is...Ch. 14 - Practice Problem 14.14
Explain how NMR...Ch. 14 - PRACTICE PROBLEM 14.15 Four benzenoid compounds,...Ch. 14 - Prob. 16PCh. 14 - Write structural formulas and give acceptable...Ch. 14 - Prob. 18PCh. 14 - Prob. 19PCh. 14 - Prob. 20PCh. 14 - Which of the hydrogen atoms shown below is more...Ch. 14 - 14.22 The rings below are joined by a double bond...Ch. 14 - Prob. 23PCh. 14 - 14.24 (a) In 1960 T. Katz (Columbia University)...Ch. 14 - Prob. 25PCh. 14 - Prob. 26PCh. 14 - 14.27 5-Chloro-1,3-cyclopentadiene (below)...Ch. 14 - Prob. 28PCh. 14 - Furan possesses less aromatic character than...Ch. 14 - 14.30 For each of the pairs below, predict...Ch. 14 - Assign structures to each of the compounds A, B,...Ch. 14 - Prob. 32PCh. 14 - Give a structure for compound F that is consistent...Ch. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - A compound (N) with the molecular formula C9H10O...Ch. 14 - The IR and 1H NMR spectra for compound X(C8H10)...Ch. 14 - Prob. 38PCh. 14 - Prob. 39PCh. 14 - 14.39 Given the following information, predict the...Ch. 14 - Consider these reactions: The intermediate A is a...Ch. 14 - Prob. 42PCh. 14 - Compound E has the spectral features given below....Ch. 14 - Draw all of the molecular orbitals for...Ch. 14 - Prob. 1LGPCh. 14 - Prob. 2LGPCh. 14 - 3. The NMR signals for the aromatic hydrogens of...Ch. 14 - Prob. 4LGPCh. 14 - Prob. 5LGPCh. 14 - Prob. 1QCh. 14 - Which is the correct name of the compound shown?...Ch. 14 - Prob. 3QCh. 14 - Prob. 4QCh. 14 - Give the structure of a compound with the formula...Ch. 14 - Prob. 6Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
4. What five specific threats to biodiversity are described in this chapter? Provide an example of each.
Biology: Life on Earth (11th Edition)
North Atlantic: distance =km100,000cm/km=cm Rate of spreading =cm/yr
Applications and Investigations in Earth Science (9th Edition)
Why are BSL-4 suits pressurized? Why not just wear tough regular suits?
Microbiology with Diseases by Body System (5th Edition)
1. Define and distinguish incomplete penetrance and variable expressivity.
Genetic Analysis: An Integrated Approach (3rd Edition)
What is the difference between transcription and translation?
Principles of Anatomy and Physiology
Two culture media were inoculated with four different bacteria. After incubation, the following results were ob...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major products of the following organic reaction: + Δ A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privaarrow_forwardesc 2 Incorrect Feedback: Your answer is incorrect. Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? A O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. . If your answer is no, check the box under the drawing area instead. Check F1 ! @ X C Save For Later Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 80 et A ད 1 4 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 # $ 45 % A 6 87 & * 8 9 ) 0 + ||arrow_forwardCan the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ?A Δ O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit ku F11arrow_forward
- १ eq ine teaching and × + rn/takeAssignment/takeCovalentActivity.do?locator-assignment-take [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is In progress mit Answer Retry Entire Group 5 more group attempts remaining Cengage Learning | Cengage Technical Support Save and Exitarrow_forwardDraw the molecules.arrow_forwardDraw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol with arrows please.arrow_forward
- . Draw the products for addition reactions (label as major or minor) of the reaction between 2-methyl-2-butene and with following reactants : Steps to follow : A. These are addition reactions you need to break a double bond and make two products if possible. B. As of Markovnikov rule the hydrogen should go to that double bond carbon which has more hydrogen to make stable products or major product. Here is the link for additional help : https://study.com/academy/answer/predict-the-major-and-minor-products-of-2-methyl- 2-butene-with-hbr-as-an-electrophilic-addition-reaction-include-the-intermediate- reactions.html H₂C CH3 H H3C CH3 2-methyl-2-butene CH3 Same structure CH3 IENCESarrow_forwardDraw everything on a piece of paper including every single step and each name provided using carbons less than 3 please.arrow_forwardTopics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. H The IUPAC name isarrow_forward
- [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardPlease draw.arrow_forwardA chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY