
(a)
Interpretation:
The numbers of chlorination products obtained from radical chlorination of methyl cyclohexane has to be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Chlorination:
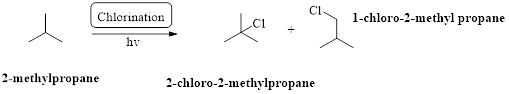
2-methylpropane undergoes radical chlorination which yields the 2-chloro-2-methylpropane and 1-chloro-2-methylpropane.
The mechanism of monochlorination of ethane (as an example) includes three steps,
- (i) Initiation
- (ii) Propagation
- (iii) Termination
The mechanism of monochlorination of ethane is shown below,
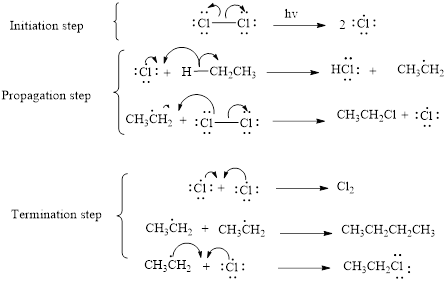
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat
(b)
Interpretation:
The number of monochlorination products obtained from the radical chlorination of methyl cyclohexane should be given when all stereoisomers are included.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Chlorination:
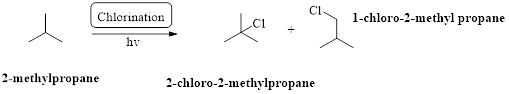
2-methylpropane undergoes radical chlorination which yields the 2-chloro-2-methylpropane and 1-chloro-2-methylpropane.
The mechanism of monochlorination of ethane (as an example) includes three steps,
- (i) Initiation
- (ii) Propagation
- (iii) Termination
The mechanism of monochlorination of ethane is shown below,

In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat
Chiral: Four different atoms attached to a carbon atom is called chiral molecule.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called enantiomers
Total number of stereoisomers = 2n
Where n is the number of chiral centers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Essential Organic Chemistry Study Guide & Solution Manual, Books a la Carte Edition
- 4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardIII O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward
- 3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forwardWhat is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forward
- Use the reaction coordinate diagram to answer the below questions. Type your answers into the answer box for each question. (Watch your spelling) Energy A B C D Reaction coordinate E A) Is the reaction step going from D to F endothermic or exothermic? A F G B) Does point D represent a reactant, product, intermediate or transition state? A/ C) Which step (step 1 or step 2) is the rate determining step? Aarrow_forward1. Using radii from Resource section 1 (p.901) and Born-Lande equation, calculate the lattice energy for PbS, which crystallizes in the NaCl structure. Then, use the Born-Haber cycle to obtain the value of lattice energy for PbS. You will need the following data following data: AH Pb(g) = 196 kJ/mol; AHƒ PbS = −98 kJ/mol; electron affinities for S(g)→S¯(g) is -201 kJ/mol; S¯(g) (g) is 640kJ/mol. Ionization energies for Pb are listed in Resource section 2, p.903. Remember that enthalpies of formation are calculated beginning with the elements in their standard states (S8 for sulfur). The formation of S2, AHF: S2 (g) = 535 kJ/mol. Compare the two values, and explain the difference. (8 points)arrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


