
Concept explainers
(a)
Interpretation:
The reaction should be completed.
Concept introduction:
Hydrobromination:
A hydrobromination reaction is one of the electrophilic additions to

(b)
Interpretation:
The reaction should be completed.
Concept introduction:
Oxidation reaction:
Alcohol undergoes oxidation reaction using oxidising agent like
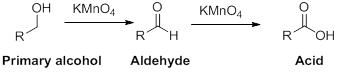
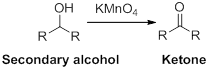
(c)
Interpretation:
The reaction should be completed.
Concept Introduction:
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
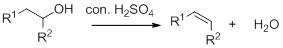
Alcohol is reaction with concentrated sulfuric acid, first alcohol gets protonated forms carbocation (more stable carbocation) followed by elimination of proton (
Tertiary carbocation is more stable than the secondary, secondary carbocation is more stable than primary.
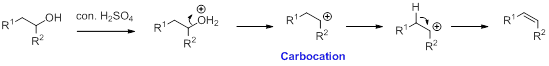 In Dehydration reaction, sulfuric acid is act as a proton donor, and which is used to protonate the alcohol and makes carbocation therefore sulfuric acid is the driving force of the reaction. Dehydration reaction will not go without acid (sulfuric acid).
In Dehydration reaction, sulfuric acid is act as a proton donor, and which is used to protonate the alcohol and makes carbocation therefore sulfuric acid is the driving force of the reaction. Dehydration reaction will not go without acid (sulfuric acid).
(d)
Interpretation:
The reaction should be completed.
Concept Introduction:
Bromination of alkenes:
Alkene undergoes bromination which yields the dibromo compound (vicinal dibromides or 1,2-dibromides).
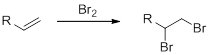
(e)
Interpretation:
The reaction should be completed.
Concept introduction:
Thiols:
The general structure of thiol is
Oxidation of thiols:
Thiol undergoes oxidation using

(f)
Interpretation:
The reaction should be completed.
Concept introduction:
Hydration:
When alkene is undergoes hydration with water in the presence of sulfuric acid which yields the alcohol. In this reaction, the water molecule will behave like a hydrogen halide to the alkene which gives the addition product this reaction is known as a hydration reaction.
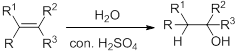
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (
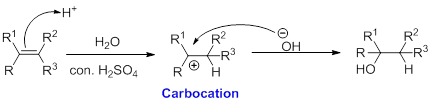
In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
(g)
Interpretation:
The reaction should be completed.
Concept introduction:
Oxidation reaction:
Alcohol undergoes oxidation reaction using oxidising agent like
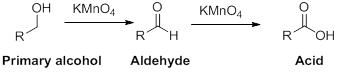
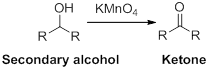
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Fundamentals of General, Organic, and Biological Chemistry, Books a la Carte Plus Mastering Chemistry with Pearson eText -- Access Card Package (8th Edition)
- Calculate pH of a solution prepared by dissolving 1.60g of sodium acetate, in 88.5 mL of 0.10 M acetic acid. Assume the volume change upon dissolving the sodium acetate is negligible. Ka is 1.75 x 10^-5arrow_forwardShow a mechanism that leads to the opening of the ring below under acid-catalyzed conditions. Give the correct Fischer projection for this sugar.arrow_forwardWhat is the stereochemical relationship between B & C?arrow_forward
- Don't use ai or any chat gpt will dislike okk just use accurate information okkk okkk just solve full accurate. don't use guidelines okk just did it accurate 100% sure experts solve it correct complete solutions okkk follow all instructions requirements okkkarrow_forwardhow would you make this plot in excel?arrow_forwardwhat is the productarrow_forward
- Balance the following equation and list of coefficients in order from left to right. SF4+H2O+—-> H2SO3+HFarrow_forwardProblem 15 of 15 Submit Using the following reaction data points, construct Lineweaver-Burk plots for an enzyme with and without an inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Using the information from this plot, determine the type of inhibitor present. 1 mM-1 1 s mM -1 [S]' V' with 10 μg per 20 54 10 36 20 5 27 2.5 23 1.25 20 Answer: |||arrow_forward12:33 CO Problem 4 of 15 4G 54% Done On the following Lineweaver-Burk -1 plot, identify the by dragging the Km point to the appropriate value. 1/V 40 35- 30- 25 20 15 10- T Км -15 10 -5 0 5 ||| 10 15 №20 25 25 30 1/[S] Г powered by desmosarrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:CengageCase Studies In Health Information ManagementBiologyISBN:9781337676908Author:SCHNERINGPublisher:Cengage
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:CengageCase Studies In Health Information ManagementBiologyISBN:9781337676908Author:SCHNERINGPublisher:Cengage





