
(a)
Interpretation:
The structure of secondary alcohol having molecular formula
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms.
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to one other carbon atom is known as primary (
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to two other carbon atoms is known as secondary (
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to three other carbon atoms is known as tertiary (
(b)
Interpretation:
The structure of ether having molecular formula
Concept Introduction:
Ether contains an oxygen atom which is linked with two alkyl groups or two aryl groups or one oxygen atom is linked with two carbon atoms by single bond.
The general structure of ether is:
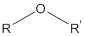
Where R and R' is alkyl or aryl groups.
(c)
Interpretation:
The structure of tertiary
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
The compounds in which hydrogen atoms of an
When the halogen is present on the carbon atom and that carbon atom is bonded to one other carbon atom is known as primary (
When the halogen is present on the carbon atom and that carbon atom is bonded to two other carbon atoms is known as secondary (
When the halogen is present on the carbon atom and that carbon atom is bonded to three other carbon atoms is known as tertiary (
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





