
Concept explainers
(a)
Interpretation: The number of peaks present in the given NMR signal of labeled proton is to be calculated.
Concept introduction: In NMR spectrum, peaks are known as resonances, lines or absorptions. The number of NMR signal in a compound is equal to the number of chemically non-equivalent protons present in that compound. In
Answer to Problem 14.17P
The labeled proton splits into septet in NMR spectrum of the given compound.
Explanation of Solution
The given compound is
Where,
➢
➢
Here,
Substitute the value of
Hence, the labeled proton gives seven peaks in NMR spectrum.
The labeled proton splits into septet in NMR spectrum of the given compound.
(b)
Interpretation: The number of peaks present in the given NMR signal of labeled proton is to be calculated.
Concept introduction: In NMR spectrum, peaks are known as resonances, lines or absorptions. The number of NMR signal in a compound is equal to the number of chemically non-equivalent protons present in that compound. In
Answer to Problem 14.17P
The labeled proton (a), (b), and (c) splits into triplet, multiplet and quintet in NMR spectrum of the given compound.
Explanation of Solution
The given compound is
Where,
➢
➢
Here,
Substitute the value of
Hence, the labeled proton (a) gives three peaks (triplet) in NMR spectrum.
Proton (b) is bonded to one methylene group that has two hydrogen atoms and one methyl group that has three hydrogen atoms. The number of peaks is calculated by the formula,
Where,
➢
➢
Here,
Substitute the value of
Hence, the labeled proton (b) splits into a multiplet in NMR spectrum.
Proton (c) is bonded to two methylene groups that have four hydrogen atoms. The number of peaks is calculated by the formula,
Where,
➢
➢
Here,
Substitute the value of
Hence, the labeled proton (c) gives quintet in NMR spectrum.
Therefore, protons (a), (b), and (c) give
The labeled proton (a), (b), and (c) splits into triplet, multiplet and quintet in NMR spectrum of the given compound.
(c)
Interpretation: The number of peaks present in the given NMR signal of labeled proton is to be calculated.
Concept introduction: In NMR spectrum, peaks are known as resonances, lines or absorptions. The number of NMR signal in a compound is equal to the number of chemically non-equivalent protons present in that compound. In
Answer to Problem 14.17P
The labeled proton (a) and (b) splits into doublet and sextet in NMR spectrum of the given compound.
Explanation of Solution
The given compound is shown below.
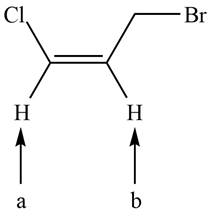
Figure 1
There are two labeled protons, (a) and (b). Proton (b) is bonded to a carbon atom that has one hydrogen and one methylene group. The number of peaks is calculated by the formula,
Where,
➢
➢
Here,
Substitute the value of
Hence, the labeled proton (b) splits into a sextet in NMR spectrum.
Proton (a) is bonded to a carbon atom that has only one hydrogen atom. The number of peaks is calculated by the formula,
Where,
➢
➢
Here,
Substitute the value of
Hence, the labeled proton (a) gives two peaks (doublet) in NMR spectrum.
Therefore, protons (a) and (b) give
The labeled proton (a) and (b) splits into doublet and sextet in NMR spectrum of the given compound.
(d)
Interpretation: The number of peaks present in the given NMR signal of labeled proton is to be calculated.
Concept introduction: In NMR spectrum, peaks are known as resonances, lines or absorptions. The number of NMR signal in a compound is equal to the number of chemically non-equivalent protons present in that compound. In
Answer to Problem 14.17P
The labeled proton (a) and (b) splits into triplet, doublet and doublet in NMR spectrum of the given compound.
Explanation of Solution
The given compound is shown below.
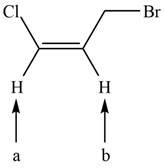
Figure 2
There are two labeled protons, (a) and (b). Proton (a) is bonded to a carbon atom that has two hydrogen atoms. The number of peaks is calculated by the formula,
Where,
➢
➢
Here,
Substitute the value of
Hence, the labeled proton (a) gives three peaks (triplet) in NMR spectrum.
Both protons (b) are bonded to a carbon atom that has only one hydrogen atom. The number of peaks is calculated by the formula,
Where,
➢
➢
Here,
Substitute the value of
Hence, the labeled protons (b) give two peaks (doublet) in NMR spectrum.
Therefore, protons (a) and (b) give
The labeled proton (a) and (b) splits into triplet, doublet and doublet in NMR spectrum of the given compound.
Want to see more full solutions like this?
Chapter 14 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Fina x | Sign X Sign X lab: X Intro X Cop) X a chat x My x Grad xLaur x Laur x a sheg X S Shoj XS SHE X acmillanlearning.com/ihub/assessment/f188d950-dd73-11e0-9572-0800200c9a66/d591b3f2-d5f7-4983-843c-0d00c1c0340b/f2b47861-07c4-4d1b-a1ee-e7db27d6b4ee?actualCourseld=d591b3f2- 5 © Macmillan Learning Organic Chemistry Maxwell presented by Macmillan Learning For the dehydrohalogenation (E2) reaction shown, draw the Zaitsev product, showing the stereochemistry clearly. H H KOH Br EtOH Heat Select Draw Templates More Erase // C H Q Search hp Q2 Q Δ קו Resouarrow_forwardIs the structural form shown possible given the pKa constraints of the side chains?arrow_forwardon x Fina X Sign X Sign x lab X Intro X Cop X chat X My x Grac x Laur x Laur x ashes x S Shox S SHE x a eve.macmillanlearning.com/ihub/assessment/f188d950-dd73-11e0-9572-0800200c9a66/d591b3f2-d5f7-4983-843c-0d00c1c0340b/f2b47861-07c4-4d1b-a1ee-e7db27d6b4ee?actualCourseld=d591b3f2-c stions estion. ct each urces. +95 Macmillan Learning Draw the product formed by the reaction of potassium t-butoxide with (15,25)-1-bromo-2-methyl-1-phenylbutane (shown). Clearly show the stereochemistry of the product. H BH (CH3)3CO-K+ +100 H3CW (CH3)3COH +85 H3CH2C +95 ossible ↓ Q Search Select Draw Templates More C H 0 bp A Erase 2Q 112 Resouarrow_forward
- Identify the structure of the PTH derivative generated after two rounds of Edman degradation.arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 31 0.5 30 26 29 22 28 100 27 33 26 23 15 4 • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. 妊 n ? Previous Nextarrow_forwardfor this question. Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 98.1106. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forward
- PLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS!!! PLEASE I UNDERSTAND THE BASICS BUT THIS IS AN EXCEPTION THAT EVEN THE INTERNET CANT HELP!!!! THIS IS THE THIRD TIME I'VE SENT THOSE QUESTIONS SO PLEASE DONT RESEND THE SAME STUFF, ITS NOT HELPING ME!!! I ALSO ALREADY TRIED TO DRAW THE MECHANISM MYSELF, SO IF ITS RIGHT PLEASE TELL ME OR TELL ME WHAT I HAVE TO CHANGE!!! First image: I have to SHOW (DRAWING) the mechanism (with arows and structures of molecules) NOT WORDS PLEASE! of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mechanism (IMAGE) (with arrows and structures of the molecules) NOT WORDS PLEASE !! for the reaction on the left, where the alcohol A is added fast in one portion HOMEWORK, NOT EXAM!! ALL DETAILS ARE IN THE IMAGES PLEASE LOOK AT THE IMAGES, DONT LOOK AT THE AI GENERATED TEXT!!!arrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forward
- Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forwardCan I please get help with this? And can I please the lowest possible significant number?arrow_forwardWhat is the molar mass of a gas that takes three times longer to effuse than helium?arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

