
Concept explainers
Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual. Study Guide, and Problems Book.
Assessing the-
Fructose-1.6-blsphosphate + H2O → Fmrlose-6-P + Pi
The human brain requires glucose as its only energy source, and the typical brain consumes about 120 g (or 480 kilocalories) of glucose dally. Ordinarily, two pieces of sausage pizza could provide more than enough potential glucose to feed the brain for a day. According to a national fast-food chain, two pieces of sausage pizza provide 1340 kilocalories. 48% of which is from fat. Fats cannot be converted to glucose in gluconeogenesis, so that leaves 697 kilocalories potentially available for glucose synthesis. The first-order rate constant for the hydrolysis of fructose-l.6-bispliosphate in the absence of enzyme is 2 × 10-20 /sec. Calculate how long it would take to provide enough glucose for one day of brain activity from two pieces of sausage pizza without the enzyme.
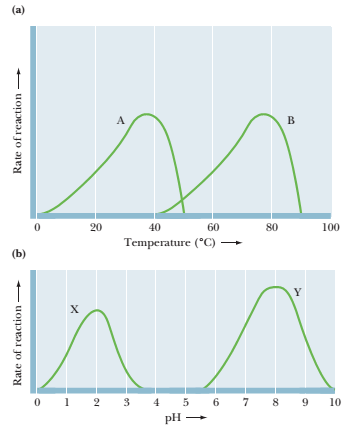
The following graphs show the temperature and pH dependencies of four enzymes, A, Î’, X, and Y. Problems 12 through IS refer to these graphs.
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
EBK BIOCHEMISTRY
- Imagine that aldolase can react with the seven carbon molecule Sedoheptulose-1,7-bisphosphate (below). Use the mechanism to predict the two products generated. Please draw out the stereochemistry in a fischer projection.arrow_forwardSodium borohydride (NaBH4) is a potent inhibitor of aldolase. It is known to ONLY inhibit theenzyme when it is complexed with substrate. Treatment of the enzyme alone has no effect.What is the mechanism for this inhibition? Please draw out the mechanism and show how it inhibits this.arrow_forwardShow the fate of the proton on the 4-Oxygen molecule of F-1,6-BP. Please include a drawing showing the electron flow that occurs.arrow_forward
- 1. Which one is the major organic product obtained from the following aldol condensation? O NaOH, H₂O heat A B C D Earrow_forwardAn organic chemist ordered the wrong item. She wanted to obtain 1-hydroxy-2-butanone, butinstead ordered 2-hydroxybutyraldehyde. As a good biochemist, show how the organic chemistcould use biological catalysis to make her desired compound. Please show the mechanism by drawing.arrow_forwardShow the fate of the hydrogen on carbon-2 of glucose. Please draw out the structure using curve arrows to show electron flow.arrow_forward
- 3. Which one of the compounds below is the major product formed by the reaction sequence shown here? CH3 + CH3NO2 NaOH H2, Ni ? nitromethane acetophenone OH OH HO HN- u x x x x Ph A HO -NH2 HO H Ph Ph Ph N- H B Ph NH2 D Earrow_forward4. Only ONE of the five compounds below can be prepared by an aldol condensation in which a single carbonyl compound is treated with base. Which one is it? To solve this problem, reverse the aldol condensation that formed each of these molecules to find out what two molecules came together to make the products. The one in which the two molecules are identical is the answer. Ph Ph ཚིག གནས ག ནཱ ཀ ན ཀནཱ A Ph H B Ph Ph H D Ph. Ph Ph E Harrow_forward5. Which one is the major organic product obtained from the following reaction sequence? First, equimolar amounts of cyclopentanone and LDA are mixed at -78°C. Then propionaldehyde (propanal) is added. Addition of aqueous acid completes the process. LDA, -78°C. 1. 2. H₂O* H A B H 0 D H H Earrow_forward
- 2. Which one is the major organic product obtained from the following reaction? NaOH, H₂O heat A B C D Earrow_forwardCH3CH2CHO + propanal PhCH2CHO 2-phenylacetaldehyde mixture of four products NaOH 10. In the crossed aldol reaction of propanal and 2-phenylacetaldehyde shown above, a mixture of four products will be formed. Which ONE of the compounds below will NOT be formed in this crossed aldol reaction? OH Ph A H OH OH Ph H B OH OH H H H Ph Ph C Ph D Earrow_forwardAn organic chemist ordered the wrong item. She wanted to obtain 1-hydroxy-2-butanone, butinstead ordered 2-hydroxybutyraldehyde. As a good biochemist, show how the organic chemistcould use biological catalysis to make her desired compound.arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
