
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.6, Problem 15P
Identify the
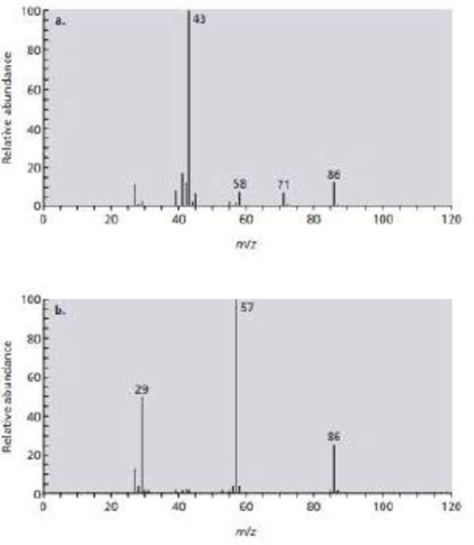
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
11
1 Which one of the following compounds would show a
proton NMR signal at the highest chemical shift? (7pts)
cl
@amitabh
CI CI
d)
Cl
CICI
None
H2SO4 (cat.), H₂O
100 °C
NH₂
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY
Ch. 13.1 - Which of the following fragments produced in a...Ch. 13.2 - What distinguishes the mass spectrum of...Ch. 13.2 - What is the most likely m/z value for the base...Ch. 13.3 - Prob. 5PCh. 13.3 - a. Suggest possible molecular formulas for a...Ch. 13.3 - If a compound has a molecular ion with an...Ch. 13.3 - Identify the hydrocarbon that has a molecular ion...Ch. 13.4 - Predict the relative intensities of the molecular...Ch. 13.5 - Which molecular formula has an exact molecular...Ch. 13.5 - Prob. 11P
Ch. 13.6 - Sketch the mass spectrum expected for...Ch. 13.6 - The mass spectra of 1-methoxybutane,...Ch. 13.6 - Primary alcohols have a strong peak at m/z = 31....Ch. 13.6 - Identify the ketones responsible for the mass...Ch. 13.6 - Prob. 16PCh. 13.6 - Using curved arrows, show the principal fragments...Ch. 13.6 - The reaction of (Z)-2-pentene with water and a...Ch. 13.9 - a. Which is higher in energy: electromagnetic...Ch. 13.9 - Prob. 20PCh. 13.13 - Prob. 21PCh. 13.14 - Which occur at a larger wavenumber: a. the C O...Ch. 13.14 - Prob. 23PCh. 13.14 - Prob. 24PCh. 13.14 - Rank the following compounds from highest...Ch. 13.14 - Which shows an O H stretch at a larger...Ch. 13.16 - Prob. 27PCh. 13.16 - a. An oxygen-containing compound shows an...Ch. 13.16 - Prob. 29PCh. 13.16 - For each of the following pair of compounds, name...Ch. 13.17 - Which of the following compounds has a vibration...Ch. 13.17 - Prob. 32PCh. 13.18 - A compound with molecular formula C4H6O gives the...Ch. 13.20 - Prob. 34PCh. 13.20 - Prob. 35PCh. 13.21 - Predict the max of the following compound:Ch. 13.21 - Prob. 37PCh. 13.23 - a. At pH = 7 one of the ions shown here is purple...Ch. 13.23 - Prob. 39PCh. 13.23 - Prob. 40PCh. 13 - In the mass spectrum of the following compounds,...Ch. 13 - Prob. 42PCh. 13 - Draw structures for a saturated hydrocarbon that...Ch. 13 - Rank the following compounds in order of...Ch. 13 - For each of the following pairs of compounds,...Ch. 13 - a. How could you use IR spectroscopy to determine...Ch. 13 - Assuming that the force constant is approximately...Ch. 13 - Norlutin and Enovid are ketones that suppress so...Ch. 13 - In the following boxes, list the types of bonds...Ch. 13 - A mass spectrum shows significant peaks at m/z. =...Ch. 13 - Prob. 51PCh. 13 - Prob. 52PCh. 13 - Prob. 53PCh. 13 - The IR spectrum of a compound with molecular...Ch. 13 - Rank the following compounds from highest...Ch. 13 - Rank the following compounds from highest...Ch. 13 - What peaks in their mass spectra can be used to...Ch. 13 - Prob. 58PCh. 13 - Which one of the following five compounds produced...Ch. 13 - Prob. 60PCh. 13 - Each of the IR spectra shown below is accompanied...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - How can IR spectroscopy distinguish between...Ch. 13 - Prob. 65PCh. 13 - Prob. 66PCh. 13 - Give approximate wavenumbers for the major...Ch. 13 - Prob. 68PCh. 13 - Which one of the following live compounds produced...Ch. 13 - Phenolphthalein is an acid-base indicator. In...Ch. 13 - Prob. 71PCh. 13 - How can you use UV spectroscopy to distinguish...Ch. 13 - Prob. 73PCh. 13 - The IR and mass spectra for three different...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- X Draw the major products of the elimination reaction below. If elimination would not occur at a significant rate, check the box under the drawing area instead. ది www. Cl + OH Elimination will not occur at a significant rate. Click and drag to start drawing a structure.arrow_forwardNonearrow_forward1A H 2A Li Be Use the References to access important values if needed for this question. 8A 3A 4A 5A 6A 7A He B C N O F Ne Na Mg 3B 4B 5B 6B 7B 8B-1B 2B Al Si P 1B 2B Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe * Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha ****** Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Analyze the following reaction by looking at the electron configurations given below each box. Put a number and a symbol in each box to show the number and kind of the corresponding atom or ion. Use the smallest integers possible. cation anion + + Shell 1: 2 Shell 2: 8 Shell 3: 1 Shell 1 : 2 Shell 2 : 6 Shell 1 : 2 Shell 2: 8 Shell 1: 2 Shell 2: 8arrow_forward
- Nonearrow_forwardIV. Show the detailed synthesis strategy for the following compounds. a. CH3CH2CH2CH2Br CH3CH2CCH2CH2CH3arrow_forwardDo the electrons on the OH participate in resonance with the ring through a p orbital? How many pi electrons are in the ring, 4 (from the two double bonds) or 6 (including the electrons on the O)?arrow_forward
- Predict and draw the product of the following organic reaction:arrow_forwardNonearrow_forwardRedraw the molecule below as a skeletal ("line") structure. Be sure to use wedge and dash bonds if necessary to accurately represent the direction of the bonds to ring substituents. Cl. Br Click and drag to start drawing a structure. : ☐ ☑ Parrow_forward
- K m Choose the best reagents to complete the following reaction. L ZI 0 Problem 4 of 11 A 1. NaOH 2. CH3CH2CH2NH2 1. HCI B OH 2. CH3CH2CH2NH2 DII F1 F2 F3 F4 F5 A F6 C CH3CH2CH2NH2 1. SOCl2 D 2. CH3CH2CH2NH2 1. CH3CH2CH2NH2 E 2. SOCl2 Done PrtScn Home End FA FQ 510 * PgUp M Submit PgDn F11arrow_forwardNonearrow_forwardPlease provide a mechanism of synthesis 1,4-diaminobenzene, start from a benzene ring.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY