
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.16, Problem 30P
For each of the following pair of compounds, name one absorption band that can be used to distinguish between them.
- a. CH3CH2CH2CH3 and CH3CH2OCH3
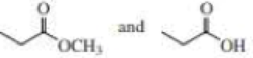
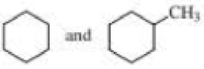
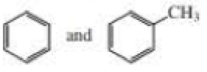
- b. CH3CH2C≡CCH3 and CH3CH2C≡CH
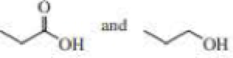
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
can you help me solve and highlight these hw
can you please help me draw these structures for HW
can you please help me draw these two structures , it is homework
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY
Ch. 13.1 - Which of the following fragments produced in a...Ch. 13.2 - What distinguishes the mass spectrum of...Ch. 13.2 - What is the most likely m/z value for the base...Ch. 13.3 - Prob. 5PCh. 13.3 - a. Suggest possible molecular formulas for a...Ch. 13.3 - If a compound has a molecular ion with an...Ch. 13.3 - Identify the hydrocarbon that has a molecular ion...Ch. 13.4 - Predict the relative intensities of the molecular...Ch. 13.5 - Which molecular formula has an exact molecular...Ch. 13.5 - Prob. 11P
Ch. 13.6 - Sketch the mass spectrum expected for...Ch. 13.6 - The mass spectra of 1-methoxybutane,...Ch. 13.6 - Primary alcohols have a strong peak at m/z = 31....Ch. 13.6 - Identify the ketones responsible for the mass...Ch. 13.6 - Prob. 16PCh. 13.6 - Using curved arrows, show the principal fragments...Ch. 13.6 - The reaction of (Z)-2-pentene with water and a...Ch. 13.9 - a. Which is higher in energy: electromagnetic...Ch. 13.9 - Prob. 20PCh. 13.13 - Prob. 21PCh. 13.14 - Which occur at a larger wavenumber: a. the C O...Ch. 13.14 - Prob. 23PCh. 13.14 - Prob. 24PCh. 13.14 - Rank the following compounds from highest...Ch. 13.14 - Which shows an O H stretch at a larger...Ch. 13.16 - Prob. 27PCh. 13.16 - a. An oxygen-containing compound shows an...Ch. 13.16 - Prob. 29PCh. 13.16 - For each of the following pair of compounds, name...Ch. 13.17 - Which of the following compounds has a vibration...Ch. 13.17 - Prob. 32PCh. 13.18 - A compound with molecular formula C4H6O gives the...Ch. 13.20 - Prob. 34PCh. 13.20 - Prob. 35PCh. 13.21 - Predict the max of the following compound:Ch. 13.21 - Prob. 37PCh. 13.23 - a. At pH = 7 one of the ions shown here is purple...Ch. 13.23 - Prob. 39PCh. 13.23 - Prob. 40PCh. 13 - In the mass spectrum of the following compounds,...Ch. 13 - Prob. 42PCh. 13 - Draw structures for a saturated hydrocarbon that...Ch. 13 - Rank the following compounds in order of...Ch. 13 - For each of the following pairs of compounds,...Ch. 13 - a. How could you use IR spectroscopy to determine...Ch. 13 - Assuming that the force constant is approximately...Ch. 13 - Norlutin and Enovid are ketones that suppress so...Ch. 13 - In the following boxes, list the types of bonds...Ch. 13 - A mass spectrum shows significant peaks at m/z. =...Ch. 13 - Prob. 51PCh. 13 - Prob. 52PCh. 13 - Prob. 53PCh. 13 - The IR spectrum of a compound with molecular...Ch. 13 - Rank the following compounds from highest...Ch. 13 - Rank the following compounds from highest...Ch. 13 - What peaks in their mass spectra can be used to...Ch. 13 - Prob. 58PCh. 13 - Which one of the following five compounds produced...Ch. 13 - Prob. 60PCh. 13 - Each of the IR spectra shown below is accompanied...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - How can IR spectroscopy distinguish between...Ch. 13 - Prob. 65PCh. 13 - Prob. 66PCh. 13 - Give approximate wavenumbers for the major...Ch. 13 - Prob. 68PCh. 13 - Which one of the following live compounds produced...Ch. 13 - Phenolphthalein is an acid-base indicator. In...Ch. 13 - Prob. 71PCh. 13 - How can you use UV spectroscopy to distinguish...Ch. 13 - Prob. 73PCh. 13 - The IR and mass spectra for three different...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please help with synthesisarrow_forward10. Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the following compound. In order to recieve full credit, you MUST SHOW YOUR WORK! H₂N CI OH CI カー 11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign R/S configurations for all stereoisomers and indicate the relationship between each as enantiomer, diastereomer, or meso. NH2 H HNH, -18arrow_forwardb) 8. Indicate whether the following carbocation rearrangements are likely to occur Please explain your rational using 10 words or less not likely to occur • The double bond is still in the Same position + Likely to oc occur WHY? -3 H3C Brave Chair Conformers. Draw the chair conformer of the following substituted cyclohexane. Peform a RING FLIP and indicate the most stable conformation and briefly explain why using 20 words or less. CI 2 -cobs ?? MUST INDICATE H -2 -2 Br EQ Cl OR AT Br H& most stable WHY? - 4arrow_forward
- CH 12 Conformational Analysis. Draw all 6 conformers (one above each letter) of the compound below looking down the indicated bond. Write the letter of the conformer with the HIGHEST and LOWEST in energies on the lines provided. NOTE: Conformer A MUST be the specific conformer of the structure as drawn below -4 NOT HOH OH 3 Conformer A: Br OH A Samo Br H 04 Br H H3 CH₂ H anti stagere Br CH clipsed H Brott H IV H MISSING 2 -2 B C D E F X 6 Conformer with HIGHEST ENERGY: 13. (1 structure LOWEST ENERGY: Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the corresponding to this name. HINT: Do not forget to indicate stereochemistry when applicable. a) ८८ 2 "Br {t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa Parent name (noname) 4 Bromo Sub = 2-methylethyl-4 Bromo nonane b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane # -2 -2arrow_forwardin the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forward
- Molecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forwardIndicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY