
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 67P
Give approximate wavenumbers for the major characteristic IR absorption bands that would be given by each of the following compounds:
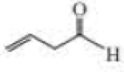
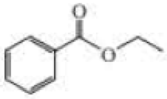
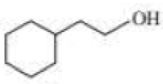
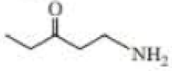
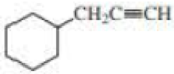
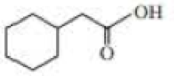
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part VII. Below are the 'HNMR, 13 C-NMR, COSY 2D- NMR, and HSQC 2D-NMR (similar with HETCOR but axes are reversed) spectra of an
organic compound with molecular formula C6H1003 - Assign chemical shift values to the H and c atoms of the
compound. Find the structure. Show complete solutions.
Predicted 1H NMR Spectrum
4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1
f1 (ppm)
Predicted 13C NMR Spectrum
100
f1 (ppm)
30
220 210 200 190 180
170
160 150 140 130 120
110
90
80
70
-26
60
50
40
46
30
20
115
10
1.0 0.9 0.8
0
-10
Q: Arrange BCC and Fec
metals, in sequence from the
Fable (Dr. R's slides) and
Calculate Volume and Density.
Aa
BCC
V
52 5
SFCC
None
Chapter 13 Solutions
EBK ORGANIC CHEMISTRY
Ch. 13.1 - Which of the following fragments produced in a...Ch. 13.2 - What distinguishes the mass spectrum of...Ch. 13.2 - What is the most likely m/z value for the base...Ch. 13.3 - Prob. 5PCh. 13.3 - a. Suggest possible molecular formulas for a...Ch. 13.3 - If a compound has a molecular ion with an...Ch. 13.3 - Identify the hydrocarbon that has a molecular ion...Ch. 13.4 - Predict the relative intensities of the molecular...Ch. 13.5 - Which molecular formula has an exact molecular...Ch. 13.5 - Prob. 11P
Ch. 13.6 - Sketch the mass spectrum expected for...Ch. 13.6 - The mass spectra of 1-methoxybutane,...Ch. 13.6 - Primary alcohols have a strong peak at m/z = 31....Ch. 13.6 - Identify the ketones responsible for the mass...Ch. 13.6 - Prob. 16PCh. 13.6 - Using curved arrows, show the principal fragments...Ch. 13.6 - The reaction of (Z)-2-pentene with water and a...Ch. 13.9 - a. Which is higher in energy: electromagnetic...Ch. 13.9 - Prob. 20PCh. 13.13 - Prob. 21PCh. 13.14 - Which occur at a larger wavenumber: a. the C O...Ch. 13.14 - Prob. 23PCh. 13.14 - Prob. 24PCh. 13.14 - Rank the following compounds from highest...Ch. 13.14 - Which shows an O H stretch at a larger...Ch. 13.16 - Prob. 27PCh. 13.16 - a. An oxygen-containing compound shows an...Ch. 13.16 - Prob. 29PCh. 13.16 - For each of the following pair of compounds, name...Ch. 13.17 - Which of the following compounds has a vibration...Ch. 13.17 - Prob. 32PCh. 13.18 - A compound with molecular formula C4H6O gives the...Ch. 13.20 - Prob. 34PCh. 13.20 - Prob. 35PCh. 13.21 - Predict the max of the following compound:Ch. 13.21 - Prob. 37PCh. 13.23 - a. At pH = 7 one of the ions shown here is purple...Ch. 13.23 - Prob. 39PCh. 13.23 - Prob. 40PCh. 13 - In the mass spectrum of the following compounds,...Ch. 13 - Prob. 42PCh. 13 - Draw structures for a saturated hydrocarbon that...Ch. 13 - Rank the following compounds in order of...Ch. 13 - For each of the following pairs of compounds,...Ch. 13 - a. How could you use IR spectroscopy to determine...Ch. 13 - Assuming that the force constant is approximately...Ch. 13 - Norlutin and Enovid are ketones that suppress so...Ch. 13 - In the following boxes, list the types of bonds...Ch. 13 - A mass spectrum shows significant peaks at m/z. =...Ch. 13 - Prob. 51PCh. 13 - Prob. 52PCh. 13 - Prob. 53PCh. 13 - The IR spectrum of a compound with molecular...Ch. 13 - Rank the following compounds from highest...Ch. 13 - Rank the following compounds from highest...Ch. 13 - What peaks in their mass spectra can be used to...Ch. 13 - Prob. 58PCh. 13 - Which one of the following five compounds produced...Ch. 13 - Prob. 60PCh. 13 - Each of the IR spectra shown below is accompanied...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - How can IR spectroscopy distinguish between...Ch. 13 - Prob. 65PCh. 13 - Prob. 66PCh. 13 - Give approximate wavenumbers for the major...Ch. 13 - Prob. 68PCh. 13 - Which one of the following live compounds produced...Ch. 13 - Phenolphthalein is an acid-base indicator. In...Ch. 13 - Prob. 71PCh. 13 - How can you use UV spectroscopy to distinguish...Ch. 13 - Prob. 73PCh. 13 - The IR and mass spectra for three different...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The emission data in cps displayed in Table 1 is reported to two decimal places by the chemist. However, the instrument output is shown in Table 2. Table 2. Iron emission from ICP-AES Sample Blank Standard Emission, cps 579.503252562 9308340.13122 Unknown Sample 343.232365741 Did the chemist make the correct choice in how they choose to display the data up in Table 1? Choose the best explanation from the choices below. No. Since the instrument calculates 12 digits for all values, they should all be kept and not truncated. Doing so would eliminate significant information. No. Since the instrument calculates 5 decimal places for the standard, all of the values should be limited to the same number. The other decimal places are not significant for the blank and unknown sample. Yes. The way Saman made the standards was limited by the 250-mL volumetric flask. This glassware can report values to 2 decimal places, and this establishes our number of significant figures. Yes. Instrumental data…arrow_forwardSteps and explanation pleasearrow_forwardSteps and explanation to undertand concepts.arrow_forward
- Nonearrow_forward7. Draw a curved arrow mechanism for the following reaction. HO cat. HCI OH in dioxane with 4A molecular sievesarrow_forwardTry: Convert the given 3D perspective structure to Newman projection about C2 - C3 bond (C2 carbon in the front). Also, show Newman projection of other possible staggered conformers and circle the most stable conformation. Use the template shown. F H3C Br Harrow_forward
- Nonearrow_forward16. Consider the probability distribution p(x) = ax", 0 ≤ x ≤ 1 for a positive integer n. A. Derive an expression for the constant a, to normalize p(x). B. Compute the average (x) as a function of n. C. Compute σ2 = (x²) - (x)², the variance of x, as a function of n.arrow_forward451. Use the diffusion model from lecture that showed the likelihood of mixing occurring in a lattice model with eight lattice sites: Case Left Right A B C Permeable Barrier → and show that with 2V lattice sites on each side of the permeable barrier and a total of 2V white particles and 2V black particles, that perfect de-mixing (all one color on each side of the barrier) becomes increasingly unlikely as V increases.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY