
Consider the reaction.
The graph below shows the concentration of
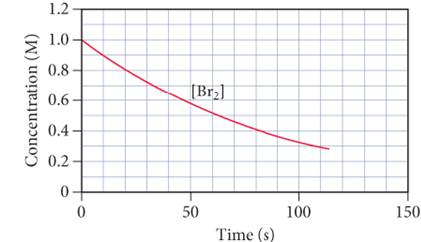
a. Use the graph to calculate:
i. The average
ii. The instantaneous rate of the reaction at 25 s.
iii. The instantaneous rate of formation of HBr at 50 s.
b. Make a rough sketch of a curve representing the concentration of HBr as a function oftime. Assume that the initial concentration ofHBr is zero.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Principles of Chemistry: A Molecular Approach (3rd Edition)
- Please answer the question and provide detailed explanations.arrow_forwardAll of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.arrow_forward5. Fill in the missing molecules in the following reaction pathway. TMSO Heat + CI then HF O₂N (1.0 equiv) AICI 3 OMearrow_forward
- e. O₂N NO2 1. excess H2, Pd/C 2. excess NaNO2, HCI 3. excess CuCNarrow_forwardHelp with a periodic table task.' Procedure Part 1: Customizing a Periodic Table Use a textbook or other valid source to determine which elements are metals, nonmetals, metalloids (called semimetals in some texts), alkali metals, alkaline earth metals, transition metals, halogens, and noble gases. Download and print a copy of the Periodic Table of Elements. Use colored pencils, colorful highlighters, or computer drawing tools to devise a schematic for designating each of the following on the periodic table: Group numbers Period number Labels for these groups: alkali metals, alkaline earth metals, transition metals, inner transition metals (lanthanides and actinides), other metals, metalloids (semimetals), other nonmetals, halogens, and noble gases Metals, nonmetals, and metalloids Note: Write the group and period numbers and color/highlight each element for categorization. Be sure to include a key for the schematic. Take a photo of the completed periodic table and upload the…arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Can you explain these two problems for mearrow_forward个 ^ Blackboard x Organic Chemistry II Lecture (m x Aktiv Learning App x → C app.aktiv.com ← Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 28 of 35 :OH H HH KO Select to Edit Arrows CH CH₂OK, CH CH2OH 5+ H :0: Donearrow_forwardCan you explain those two problems for me please.arrow_forward
- Do we need to draw the "ethyne" first for this problem? im confusedarrow_forwardCan you explain how this problem was solved.arrow_forwardQuestion 2 show work. don't Compound give Ai generated solution So (J K-1 mol-1) A 26 B 54 C 39 D 49 At 298 K, AG° is 375 kJ for the reaction 1A + 1B → 4C + 2D Calculate AH° for this reaction in kJ.arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





