
Concept explainers
The following table lists the concentrations of the principal ions in seawater:
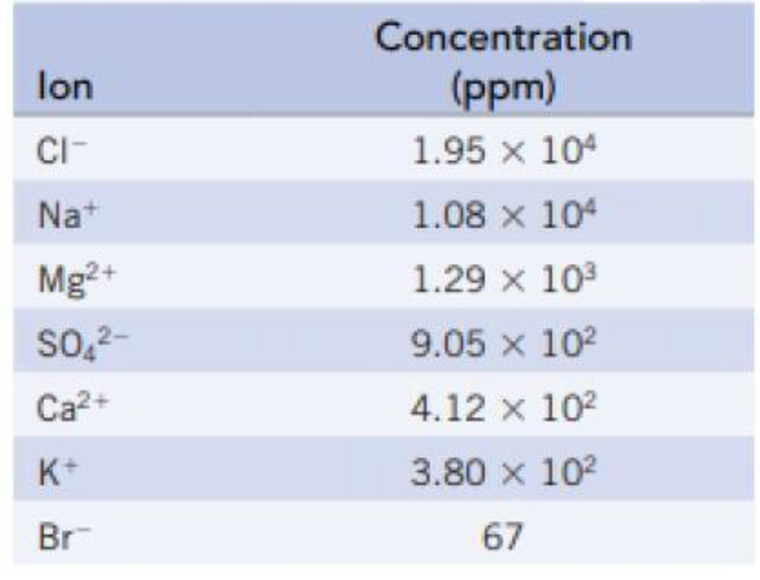
- (a) Calculate the freezing point of seawater.
- (b) Calculate the osmotic pressure of seawater at 25 °C. What is the minimum pressure needed to purify seawater by reverse osmosis?
(a)
Interpretation: The freezing point of seawater has to be determined.
Concept introduction:
Colligative properties: Properties of solutions which having influence on the concentration of the solute in it. Colligative properties are,
- Decrease in the vapor pressure
- Increase in the boiling point
- Decline in the freezing point
- Osmotic pressure
Freezing point depression: The freezing point of the solution varies with the solute concentration.
The number of moles of any substance can be determined using the equation
Answer to Problem 79GQ
Freezing point of seawater is
Explanation of Solution
Given,
Molal freezing point depression constant of water is
The value
Hence, the concentration given in ppm can be taken as the mass of each of the ions on
The number of moles of any substance can be determined using the equation
Number of moles of
Number of moles of
Number of moles of
Number of moles of
Number of moles of
Number of moles of
Number of moles of
So the total moles of principle ions in
Molality of seawater is,
Depression in freezing point is,
Therefore,
Freezing point of seawater is,
Freezing point of seawater is
(b)
Interpretation: The osmotic pressure of seawater at
Concept introduction:
Colligative properties: Properties of solutions which having influence on the concentration of the solute in it. Colligative properties are,
- Decrease in the vapor pressure
- Increase in the boiling point
- Decline in the freezing point
- Osmotic pressure
Osmotic pressure: The pressure created by the column of solution for the system at equilibrium is a measure of the osmotic pressure and is calculated by using the equation,
where,
c is the molar concentration
The number of moles of any substance can be determined using the equation
Answer to Problem 79GQ
The osmotic pressure of seawater at
Explanation of Solution
Given,
The molarity of the solute is
The osmotic pressure of seawater is,
The osmotic pressure of seawater at
To purify the seawater using reverse osmosis method, there should be a minimum pressure of
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry & Chemical Reactivity
Additional Science Textbook Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Physical Science
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Biology (11th Edition)
Organic Chemistry
- The following 'H NMR spectrum was taken with a 750 MHz spectrometer: 1.0 0.5 0.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 ' 2.0 1.0 0.0 (ppm) What is the difference Av in the frequency of RF ac Δν ac radiation absorbed by the a and c protons? (Note: it's not equal to the difference in chemical shifts.) Round your answer to 2 significant digits, and be sure it has an appropriate unit symbol. = O O a will shift left, c will shift right. O a will shift right, c will shift left. a and c will both shift left, with more space between them. Suppose a new spectrum is taken with a 500 MHz spectrometer. What will be true about this new spectrum? O a and c will both shift left, with less space between them. O a and c will both shift right, with more space between them. O a and c will both shift right, with less space between them. Which protons have the largest energy gap between spin up and spin down states? O None of the above. ○ a Ob Explanation Check C Ar B 2025 McGraw Hill LLC. All Rights Reserved.…arrow_forwardWhat mass of Na2CO3 must you add to 125g of water to prepare 0.200 m Na2CO3? Calculate mole fraction of Na2CO3, mass percent, and molarity of the resulting solution. MM (g/mol): Na2CO3 105.99; water 18.02. Final solution density is 1.04 g/mL.arrow_forward(ME EX2) Prblms Can you please explain problems to me in detail, step by step? Thank you so much! If needed color code them for me.arrow_forward
- Experiment #8 Electrical conductivity & Electrolytes. Conductivity of solutions FLINN Scientific Scale RED LED Green LED LED Conductivity 0 OFF OFF 1 Dim OFF 2 medium OFF 3 Bright Dim Low or Nowe Low Medium High 4 Very Bright Medium nd very high AA Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ Δ SE=Strong Electrolyte, FE = Fair Electrolyte CWE = Weak Electrolyte, NE= Noni Electrolyte, #Solutions 1 0.1 M NaCl 2/1x 102 M NaCl, 3/1X103 M Nall Can Prediction M Observed Conductivity Very bright red Bright red Dim red you help me understand how I'm supposed to find the predictions of the following solutions? I know this is an Ionic compound and that the more ions in a solution means it is able to carry a charge, right? AAAA Darrow_forward(SE EX 2) Prblsm 4-7: Can you please explain problems 4-7 and color code if needed for me. (step by step) detail explanationsarrow_forward(SE EX 2) Problems 8-11, can you please explain them to me in detail and color-code anything if necessary?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





