
EP BASIC CHEMISTRY-STANDALONE ACCESS
6th Edition
ISBN: 9780134999890
Author: Timberlake
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 58UTC
Interpretation Introduction
Interpretation:
‘Reaction is exothermic or endothermic’ should be identified.
Concept introduction:
Equilibrium is the condition at which the rate of formation of product is equal to rate of disappearance of reactant.
Exothermic reaction, are the reaction which liberates energy and the temperature of the product is lower than reactants to proceed the reaction.
Endothermic reaction, are the reaction which absorbs energy and the temperature of the product is higher than reactants to proceed the reaction.
Given:
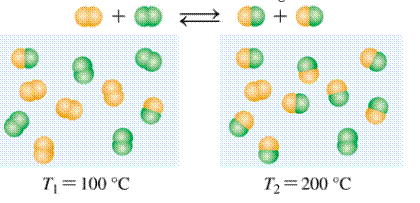
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the structure of the pound in the provided
CO
as a 300-1200
37(2), 11 ( 110, and 2.5 (20
Please help me with # 4 and 5. Thanks in advance!
A small artisanal cheesemaker is testing the acidity of their milk
before it coagulates. During fermentation, bacteria produce lactic
acid (K₁ = 1.4 x 104), a weak acid that helps to curdle the milk and
develop flavor. The cheesemaker has measured that the developing
mixture contains lactic acid at an initial concentration of 0.025 M.
Your task is to calculate the pH of this mixture and determine whether
it meets the required acidity for proper cheese development. To
achieve the best flavor, texture and reduce/control microbial growth,
the pH range needs to be between pH 4.6 and 5.0.
Assumptions:
Lactic acid is a monoprotic acid
H
H
:0:0:
H-C-C
H
:0:
O-H
Figure 1: Lewis Structure for Lactic Acid
For simplicity, you can use the generic formula HA to represent the acid
You can assume lactic acid dissociation is in water as milk is mostly water.
Temperature is 25°C
1. Write the K, expression for the dissociation of lactic acid in the space provided. Do not forget to
include state symbols.…
Chapter 13 Solutions
EP BASIC CHEMISTRY-STANDALONE ACCESS
Ch. 13.1 - Prob. 1PPCh. 13.1 - Prob. 2PPCh. 13.1 - In the following reaction, what happens to the...Ch. 13.1 - Prob. 4PPCh. 13.1 - Prob. 5PPCh. 13.1 - Prob. 6PPCh. 13.2 - What is meant by the term reversible reaction?Ch. 13.2 - Prob. 8PPCh. 13.2 - Which of the following are at equilibrium? a. The...Ch. 13.2 - Which of the following are not at equilibrium? a....
Ch. 13.2 - 13.11 The following diagrams show the chemical...Ch. 13.2 - Prob. 12PPCh. 13.3 - Write the equilibrium expression for each of the...Ch. 13.3 - Prob. 14PPCh. 13.3 - Prob. 15PPCh. 13.3 - Prob. 16PPCh. 13.3 - What is the numerical value of Kc for the...Ch. 13.3 - What is the numerical value of Kc for the...Ch. 13.3 - What is the numerical value of Kc for the...Ch. 13.3 - What is the numerical value of Kc for the...Ch. 13.3 - Prob. 21PPCh. 13.3 - Identify each of the following as a homogeneous or...Ch. 13.3 - Prob. 23PPCh. 13.3 - Prob. 24PPCh. 13.3 - Prob. 25PPCh. 13.3 - What is the numerical value of Kc for the...Ch. 13.4 - Prob. 27PPCh. 13.4 - Prob. 28PPCh. 13.4 - Indicate whether each of the following equilibrium...Ch. 13.4 - Indicale whether each of the following equilibrium...Ch. 13.4 - Prob. 31PPCh. 13.4 - The numerical value of the equilibrium constant,...Ch. 13.4 - Prob. 33PPCh. 13.4 - The numerical value of the equilibrium constant,...Ch. 13.5 - In the lower atmosphere, oxygen is converted to...Ch. 13.5 - Prob. 36PPCh. 13.5 - Hydrogen chloride can be made by reacting hydrogen...Ch. 13.5 - When heated, carbon monoxide reacts with water to...Ch. 13.5 - Use the following equation for the equilibrium of...Ch. 13.5 - Use the following equation for the equilibrium of...Ch. 13.5 - Prob. 41PPCh. 13.5 - Prob. 42PPCh. 13.6 - For each of the following slightly soluble ionic...Ch. 13.6 - For each of the following slightly soluble ionic...Ch. 13.6 - Prob. 45PPCh. 13.6 - Prob. 46PPCh. 13.6 - A saturated solution of silver carbonate, Ag2CO3 ,...Ch. 13.6 - Prob. 48PPCh. 13.6 - Calculate the molar solubility, S , of CuI if it...Ch. 13.6 - Calculate the molar solubility, S , of SnS if it...Ch. 13.6 - The CO2 level in the atmosphere has increased over...Ch. 13.6 - Prob. 52PPCh. 13 - Write the equilibrium expression for each of the...Ch. 13 - Write the equilibrium expression for each of the...Ch. 13 - Prob. 55UTCCh. 13 - Would the equilibrium constant, Ke , for the...Ch. 13 - Prob. 57UTCCh. 13 - Prob. 58UTCCh. 13 - Prob. 59APPCh. 13 - Prob. 60APPCh. 13 - For each of the following reactions, indicate if...Ch. 13 - For each of the following reactions, indicate if...Ch. 13 - Consider the reaction: (13.3) 2NH3(g)N2(g)+3H2(g)...Ch. 13 - Prob. 64APPCh. 13 - Prob. 65APPCh. 13 - Prob. 66APPCh. 13 - Prob. 67APPCh. 13 - According to Le Châtelier's principle, does the...Ch. 13 - Prob. 69APPCh. 13 - Prob. 70APPCh. 13 - The numerical value of the equilibrium constant,...Ch. 13 - The numerical value of the equilibrium constant,...Ch. 13 - For each of the following slightly soluble ionic...Ch. 13 - For each of the following slightly soluble ionic...Ch. 13 - Prob. 75APPCh. 13 - Prob. 76APPCh. 13 - Prob. 77APPCh. 13 - Prob. 78APPCh. 13 - What is the molar solubility, S , of CdS if it has...Ch. 13 - Prob. 80APPCh. 13 - Prob. 81CPCh. 13 - Prob. 82CPCh. 13 - Prob. 83CPCh. 13 - Indicate how each of the following will affect the...Ch. 13 - Prob. 85CPCh. 13 - Prob. 86CPCh. 13 - Prob. 87CPCh. 13 - Prob. 88CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :0: :0 H. 0:0 :0: :6: S: :0: Select to Edit Arrows ::0 Select to Edit Arrows H :0: H :CI: Rotation Select to Edit Arrows H. < :0: :0: :0: S:arrow_forward3:48 PM Fri Apr 4 K Problem 4 of 10 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Mg. :0: Select to Add Arrows :0: :Br: Mg :0: :0: Select to Add Arrows Mg. Br: :0: 0:0- Br -190 H 0:0 Select to Add Arrows Select to Add Arrows neutralizing workup H CH3arrow_forwardIarrow_forward
- Draw the Markovnikov product of the hydrobromination of this alkene. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for this problem. + Explanation Check 1 X E 4 1 1 1 1 1 HBr Click and drag to start drawing a structure. 80 LE #3 @ 2 $4 0 I அ2 % 85 F * K M ? BH 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center & 6 27 FG F10 8 9 R T Y U D F G H P J K L Z X C V B N M Q W A S H option command H command optiarrow_forwardBe sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. Predict the major products of the following reaction. Explanation Q F1 A Check F2 @ 2 # 3 + X 80 F3 W E S D $ 4 I O H. H₂ 2 R Pt % 05 LL ee F6 F5 T <6 G Click and drag to start drawing a structure. 27 & A 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Acce Y U H DII 8 9 F10 4 J K L Z X C V B N M T H option command F11 P H commandarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore inorganic byproducts. H :0: CH3 O: OH Q CH3OH2+ Draw Intermediate protonation CH3OH CH3OH nucleophilic addition H Draw Intermediate deprotonation :0: H3C CH3OH2* protonation H 0: H CH3 H.arrow_forward
- Predicting the reactants or products of hemiacetal and acetal formation uentify the missing organic reactants in the following reaction: H+ X+Y OH H+ за Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Explanation Check Click and drag to start drawing a structure. ? olo 18 Ar © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardcan someone please answer thisarrow_forwardPlease, please help me figure out the the moles, molarity and Ksp column. Step by step details because I've came up with about three different number and have no idea what I'm doing wrong.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY