
Biochemistry
6th Edition
ISBN: 9781305577206
Author: Reginald H. Garrett, Charles M. Grisham
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 4P
Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book.
Graphing the Results from Kinetics Experiments with Enzyme Inhibitors The following kinetic data were obtained for an enzyme in the absence of any inhibitor (1), and in the presence of two different inhibitors (2) and (3) at 5 mM concentration. Assume [ET] is the same in each experiment.
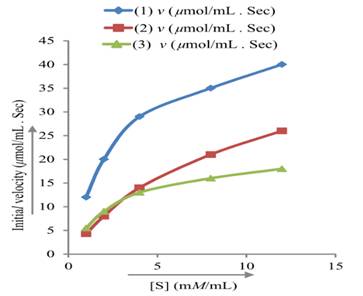
Graph these data as Lineweaver-Burk plots and use your graph to find answers to a. and b.
a. Determine Vmax and Km for the enzyme.
b. Determine the type of inhibition and the K1 for each inhibitor.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
1. What is the isoelectric point of glutamic acid?
(Glutamic acid has pKa1 2.10, pKa2 4.07, pKaз 9.47)
A) pH 2.1
D) pH 6.8
B) pH 3.1
C) pH 4.1
E) pH 9.5
HO
NH2
Glutamic acid
(shown without charges)
OH
Br
Mg, ether
1. HCHO
(formaldehyde)
2. H+, H₂O
PCC
1. NH3, HCN
?
(pyridinium chlorochromate)
2. H2O, HCI
11. Which one of the following compounds is the major organic product of the
series of reactions shown above?
Ph.
Ph.
OH
NH2₂
A
Ph.
Ή
NH2
B
OH
Ph
Η
Ph
OH
NH2
NH2₂
NH₂
C
D
E
B
A
6. Which ONE of the
labeled bonds in the
tripeptide on the right
is a peptide bond:
H₂N
N
'N'
OH
C
H
A, B, C, D or E?
HN
E
OH
Chapter 13 Solutions
Biochemistry
Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...
Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Answers to all problems are at the end of this...Ch. 13 - Prob. 15PCh. 13 - Prob. 16PCh. 13 - Prob. 17PCh. 13 - Prob. 18PCh. 13 - Answers to all problems are at the end of this...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Questions 8-9 are 0.4 points each. The next two questions relate to the peptide whose structure is shown here. To answer these questions, you should look at a table of H2N/.. amino acid structures. You don't have to memorize the structures of the amino acids. IZ 8. What is the N-terminal amino acid of this peptide? A) proline B) aspartic acid C) threonine 9. What is the C-terminal amino acid of this peptide? A) proline B) aspartic acid C) threonine N OH D) valine E) leucine D) valine E) leucine NH "OH OHarrow_forward7. What is the correct name of the following tripeptide? A) Ile-Met-Ser B) Leu-Cys-Thr C) Val-Cys-Ser D) Ser-Cys-Leu E) Leu-Cys-Ser H₂N!!!!! N H ΖΙ .SH SF H IN OH OHarrow_forwardPlease draw out the following metabolic pathways: (Metabolic Map) Mitochondrion: TCA Cycle & GNG, Electron Transport, ATP Synthase, Lipolysis, Shuttle Systems Cytoplasm: Glycolysis & GNG, PPP (Pentose Phosphate Pathway), Glycogen, Lipogenesis, Transporters and Amino Acids Control: Cori/ Glc-Ala cycles, Insulin/Glucagon Reg, Local/Long Distance Regulation, Pools Used Correctlyarrow_forward
- Please help provide me an insight of what to draw for the following metabolic pathways: (Metabolic Map) Mitochondrion: TCA Cycle & GNG, Electron Transport, ATP Synthase, Lipolysis, Shuttle Systems Cytoplasm: Glycolysis & GNG, PPP (Pentose Phosphate Pathway), Glycogen, Lipogenesis, Transporters and Amino Acids Control: Cori/ Glc-Ala cycles, Insulin/Glucagon Reg, Local/Long Distance Regulation, Pools Used Correctlyarrow_forwardwrite ionization equilibriumarrow_forwardwrite the ionization equilibrium for cysteine and calculate the piarrow_forward
- please answerarrow_forwardf. The genetic code is given below, along with a short strand of template DNA. Write the protein segment that would form from this DNA. 5'-A-T-G-G-C-T-A-G-G-T-A-A-C-C-T-G-C-A-T-T-A-G-3' Table 4.5 The genetic code First Position Second Position (5' end) U C A G Third Position (3' end) Phe Ser Tyr Cys U Phe Ser Tyr Cys Leu Ser Stop Stop Leu Ser Stop Trp UCAG Leu Pro His Arg His Arg C Leu Pro Gln Arg Pro Leu Gin Arg Pro Leu Ser Asn Thr lle Ser Asn Thr lle Arg A Thr Lys UCAG UCAC G lle Arg Thr Lys Met Gly Asp Ala Val Gly Asp Ala Val Gly G Glu Ala UCAC Val Gly Glu Ala Val Note: This table identifies the amino acid encoded by each triplet. For example, the codon 5'-AUG-3' on mRNA specifies methionine, whereas CAU specifies histidine. UAA, UAG, and UGA are termination signals. AUG is part of the initiation signal, in addition to coding for internal methionine residues. Table 4.5 Biochemistry, Seventh Edition 2012 W. H. Freeman and Company B eviation: does it play abbreviation:arrow_forwardAnswer all of the questions please draw structures for major productarrow_forward
- for glycolysis and the citric acid cycle below, show where ATP, NADH and FADH are used or formed. Show on the diagram the points where at least three other metabolic pathways intersect with these two.arrow_forwardanswer the questions please all of them should be answeredarrow_forwardBurk plot is shown below. Calculate Km and max for this enzyme. show workarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning
Chapter 7 - Human Movement Science; Author: Dr. Jeff Williams;https://www.youtube.com/watch?v=LlqElkn4PA4;License: Standard youtube license