
(a)
Interpretation:
The statement “
(a)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
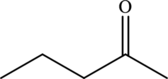
Hence, the statement
(b)
Interpretation:
The statement “
(b)
Answer to Problem 4MCP
The given statement is false because
Explanation of Solution
Alcohols have strong intermolecular hydrogen bonding. The
Ketones have lower boiling points when compared to alcohols, representing the presence of weak intermolecular dipole-dipole forces. Due to weaker intermolecular hydrogen and weaker dipole-dipole attractions in ketones, they have lower boiling points compared to alcohols.
Hence, the given statement is false because
(c)
Interpretation:
The statement “
(c)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
(d)
Interpretation:
The statement “
(d)
Answer to Problem 4MCP
The given statement is false because
Explanation of Solution
The structure of
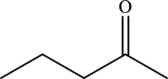
The carbonyl group present in
The statement “
(e)
Interpretation:
The statement “
(e)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
The structure of
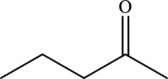
From the structure of
The statement “
(f)
Interpretation:
The statement “
(f)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
The presence of polar carbonyl group and the attractive forces between the carbonyl-containing compounds include London dispersion forces between the hydrocarbon chains and dipole-dipole attractions between the carbonyl groups. Because the dipole-dipole attractions between the molecules are stronger than London dispersion forces,
(g)
Interpretation:
The statement “
(g)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
(h)
Interpretation:
The statement “
(h)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Ketones cannot form hydrogen bonds with each other, but they form intermolecular hydrogen bonds with water. As a result, the smaller members of ketones (five or fewer carbon atoms) are soluble in water. Hence, the statement “
(i)
Interpretation:
The statement “
(i)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Ketone is carbonyl compounds. The carbonyl group of the ketone is polar and can participate in dipole-dipole attractions.
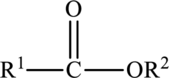
The structure of
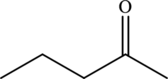
(j)
Interpretation:
The statement “
(j)
Answer to Problem 4MCP
The given statement is true.
Explanation of Solution
Structural isomers are those with same molecular formula but differ in arrangement of atoms.
The molecular formula of
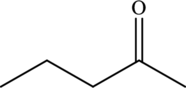
The molecular formula of pentanal is

Both these are structural isomers with each other and hence, the statement “
Want to see more full solutions like this?
Chapter 13 Solutions
GENERAL,ORGANIC,+BIOCHEM.(LL) >CUSTOM<
- The reaction is carried out with gases: A → B + C at 300 K. The total pressure is measured as a function of time (table). If the reaction order is 2, calculate the rate or kinetic constant k (in mol-1 L s¹) Ptotal (atm) 492 676 760 808 861 t(s) 0 600 1200 1800 3000arrow_forwardcan someone give a description of this NMR including whether its a triplt singlet doublet where the peak is around at ppm and what functional group it representsarrow_forward1. Determine the relationship between the following molecules as identical, diastereomers, or enantiomers (6 points, 2 points each). OH OH OH A-A OH HOT HO- ACHN and HO- ACHN OH HO HO ° OH and OH OH SH and ...SHarrow_forward
- 20,0 Complete the electron pushing mechanism to y drawing the necomery unicaciones and carved on for Step 1: Add curved arms for the tint step, traiment with NalilĻ. The Nation 458 Step 2: Added for the second step, inalment with), how the "counterion bar Step 3: Daw the products of the last simplom organic and one incoganic spacient, including all nonbondingarrow_forwardplease provide the structure for this problem, thank you!arrow_forwardDraw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forward
- please provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forwardReaction A 0,0arrow_forward
- presented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





