
Interpretation: The product formed after reaction of cyclohexane in the presence of
Concept introduction:
Molecules that have one unpaired electron are called free radicals.
Answer to Problem 1PP
Solution:

Explanation of Solution
Given information:
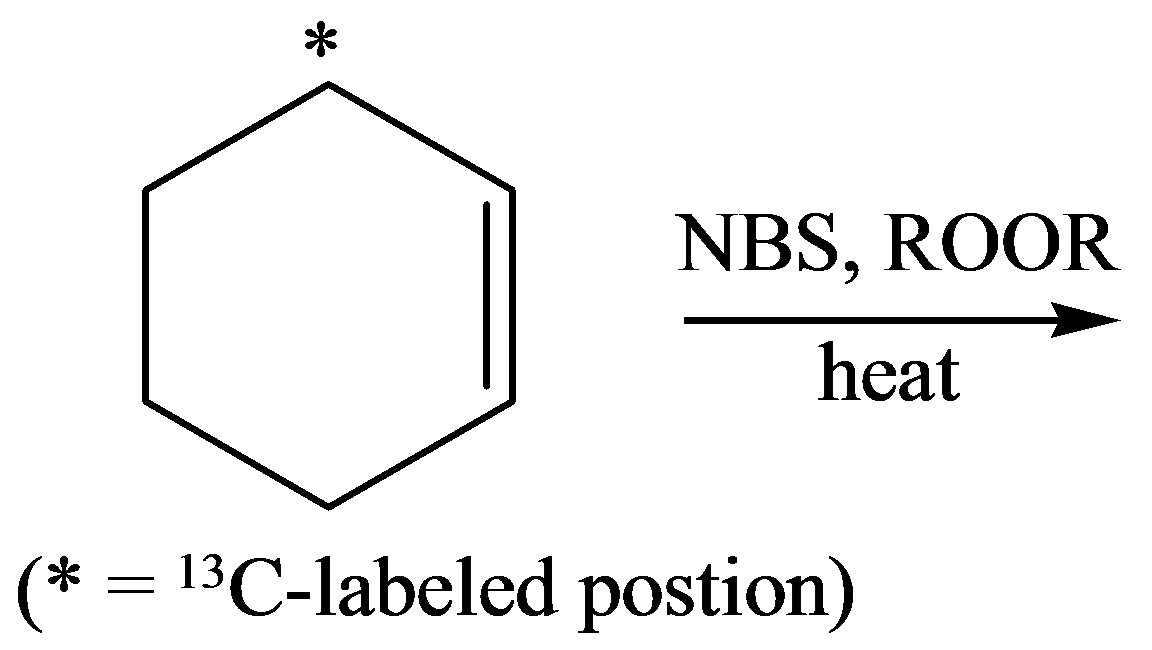
The reaction in the presence of
Allylic bromination is a free radical substitution reaction in which removal of hydrogen atom on carbon adjacent to a double bond with bromine.
Free radical substitution reaction takes place in the presence of
The structure of 3-bromocyclohexence is as follows:

The product formed after free radical substitution is 3-bromocyclohexence.
Want to see more full solutions like this?
Chapter 13 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

