
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
12th Edition
ISBN: 9781119664635
Author: Solomons
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 4PP
Practice Problem 13.4
From each set of resonance structures that follow, designate the one that would contribute most to the hybrid and explain your choice:
(a)
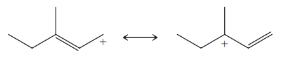
(b)
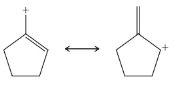
(c)
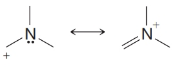
(d)
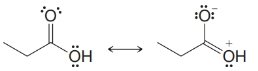
(e)

(f)

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.
Draw mechanism
Does Avogadro's number have units?
Chapter 13 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
In mechanism, photophosphorylation is most similar to A. substrate-level phosphorylation in glycolysis. B. oxid...
Campbell Biology in Focus (2nd Edition)
The following data were obtained from a disk-diffusion test. Antibiotic Zone of Inhibition A 15 mm B 0 mm c 7 m...
Microbiology: An Introduction
30. Drosophila has a diploid chromosome number of 2n = 8, which includes one pair of sex chromosomes (XX in fem...
Genetic Analysis: An Integrated Approach (3rd Edition)
47. A 250.0-mL buffer solution is 0.250 M in acetic acid and 0.250 M in sodium acetate.
a. What is the initial ...
Chemistry: A Molecular Approach (4th Edition)
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Describe the various layering arrangements and cell shapes of epithelial tissue.
Principles of Anatomy and Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forward
- Indicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forward
- Name the following molecules using iupacarrow_forwardWrite the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY