
Concept explainers
(a)
Interpretation:
The compound that is more soluble in water should be determined.
CH3 OH or CH3 OCH3.
Concept Introduction:
Water solubility is known as the measurement of the chemical substance which can dissolve into the water at the specific temperature. The solubility’s unit is mg/L or ppm i.e. parts per million. Water solubility is the most vital properties which affects the environmental fate and bioavailability of the chemical substances.
(b)
Interpretation:
The compound that is more soluble in water should be determined.
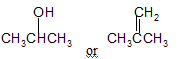
Concept Introduction:
Water solubility is known as the measurement of the chemical substance which can dissolve into the water at the specific temperature. The solubility’s unit is mg/L or ppm i.e. parts per million. Water solubility is the most vital properties which affects the environmental fate and bioavailability of the chemical substances.
(c)
Interpretation:
The compound that is more soluble in water should be determined.
CH3 CH2 CH2 SH or CH3 CH2 CH2 OH.
Concept Introduction:
Water solubility is known as the measurement of the chemical substance which can dissolve into the water at the specific temperature. The solubility’s unit is mg/L or ppm i.e. parts per million. Water solubility is the most vital properties which affects the environmental fate and bioavailability of the chemical substances.
(d)
Interpretation:
The compound that is more soluble in water should be determined.
CH3 CH2 Cl or NaCl.
Concept Introduction:
Water solubility is known as the measurement of the chemical substance which can dissolve into the water at the specific temperature. The solubility’s unit is mg/L or ppm i.e. parts per million. Water solubility is the most vital properties which affects the environmental fate and bioavailability of the chemical substances.
(e)
Interpretation:
The compound that is more soluble in water should be determined.
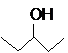 or
or 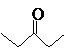
Concept Introduction:
Water solubility is known as the measurement of the chemical substance which can dissolve into the water at the specific temperature. The solubility’s unit is mg/L or ppm i.e. parts per million. Water solubility is the most vital properties which affects the environmental fate and bioavailability of the chemical substances.
Trending nowThis is a popular solution!

Chapter 13 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
