
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.54P
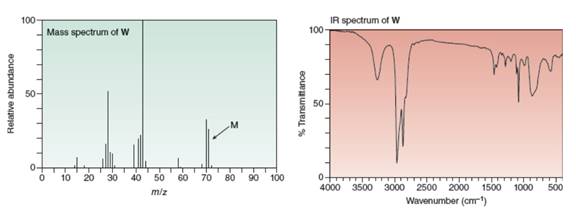
Reaction of
gives the IR and mass spectra shown below. Propose a structure for
a stepwise mechanism that accounts for its formation.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Give the mechanism and product for the reaction below. Hint - the product contains
two six membered rings
NaOEt (Base)
C
Applications of metal clusters.
Give the product and mechanism for the reaction below. Explain why this reaction is
of significance in Forensic Chemistry
HO
Heat
B
Chapter 13 Solutions
Organic Chemistry-Package(Custom)
Ch. 13 - What is the mass of the molecular ion formed from...Ch. 13 - Prob. 13.2PCh. 13 - Prob. 13.3PCh. 13 - What molecular ions would you expect for the...Ch. 13 - The mass spectrum of 2,3-dimethylpentane also...Ch. 13 - The base peak in the mass spectrum of 2, 2,...Ch. 13 - (a) What mass spectral fragments are formed by ...Ch. 13 - What cations are formed in the mass spectrometer...Ch. 13 - The low-resolution mass spectrum of an unknown...Ch. 13 - Benzene, toluene, and p-xylene BTX are often added...
Ch. 13 - Prob. 13.11PCh. 13 - Prob. 13.12PCh. 13 - Prob. 13.13PCh. 13 - Prob. 13.14PCh. 13 - How do the IR spectra of the isomers cyclopentane...Ch. 13 - How do the three isomers of molecular formula...Ch. 13 - Problem 13.18 What functional groups are...Ch. 13 - Problem-13.19 What are the major IR absorptions in...Ch. 13 - Problem-13.20 What are the major IR absorptions in...Ch. 13 - Prob. 13.20PCh. 13 - Problem-13.22 Propose structures consistent with...Ch. 13 - 13.23 What major IR absorptions are present above ...Ch. 13 - Problem-13.24 The mass spectrum of the following...Ch. 13 - What molecular ion is expected for each compound?Ch. 13 - Which compound gives a molecular ion at m/z= 122,...Ch. 13 - Propose two molecular formulas for each molecular...Ch. 13 - Propose four possible structures for a hydrocarbon...Ch. 13 - Match each structure to its mass spectrum. a. b....Ch. 13 - 13.32 Propose two possible structures for a...Ch. 13 - 13.33 What cations are formed in the mass...Ch. 13 - 13.35 For each compound, assign likely...Ch. 13 - Prob. 13.32PCh. 13 - 13.37 Propose a structure consistent with each...Ch. 13 - 13.38 A low-resolution mass spectrum of the...Ch. 13 - Can the exact mass obtained in a high-resolution...Ch. 13 - 13.39 Primary alcohols often show a peak in their...Ch. 13 - 13.40 Like alcohols, ethers undergo α cleavage by...Ch. 13 - Which of the highlighted bonds absorbs at higher v...Ch. 13 - What major IR absorptions are present above...Ch. 13 - How would each of the following pairs of compounds...Ch. 13 - 13.44 Morphine, heroin, and oxycodone are three...Ch. 13 - Prob. 13.42PCh. 13 - 13.47 Match each compound to its IR spectrum
Ch. 13 - 13.48 Propose possible structures consistent with...Ch. 13 - A chiral hydrocarbon X exhibits a molecular ion at...Ch. 13 - 13.50 A chiral compound has a strong absorption...Ch. 13 - 13.51 Treatment of benzoic acid with followed by...Ch. 13 - 13.52 Treatment of benzaldehyde with in aqueous ...Ch. 13 - Prob. 13.49PCh. 13 - 13.54 Reaction of 2-methylpropanoic acid with ...Ch. 13 - 13.55 Reaction of pentanoyl chloride with lithium...Ch. 13 - Prob. 13.52PCh. 13 - 13.57 Treatment of anisole with and forms P,...Ch. 13 - 13.58 Reaction of with forms compound ,...Ch. 13 - Problem-13.59 The carbonyl absorption of an amide...Ch. 13 - Prob. 13.56PCh. 13 - Problem-13.61 Explain why a ketone carbonyl...Ch. 13 - 13.62 Oxidation of citronellol, a constituent of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. A gene is a segment of DNA that has the information to produce a functional product. The functional product ...
Genetics: Analysis and Principles
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Metal clusters and catalysis.arrow_forwardQ1: Draw a valid Lewis structures for the following molecules. Include appropriate charges and lone pair electrons. If there is more than one Lewis structure available, draw the best structure. NH3 Sulfate Boron tetrahydride. C3H8 (linear isomer) OCN NO3 CH3CN SO2Cl2 CH3OH2*arrow_forwardIn the following molecule, indicate the hybridization and shape of the indicated atoms. -z: CH3 CH3 H3C HO: CI: :arrow_forward
- Q3: Draw the Lewis structures for nitromethane (CH3NO2) and methyl nitrite (CH3ONO). Draw at least two resonance forms for each. Determine which form for each is the major resonance contributor. Page 1 of 4 Chem 0310 Organic Chemistry 1 Recitations Q4: Draw the Lewis structures for the cyanate ion (OCN) and the fulminate ion (CNO-). Draw all possible resonance structures for each. Determine which form for each is the major resonance contributor.arrow_forwardMetallic clusters and nanomaterials.arrow_forwardMetal clusters: photochemical properties of special relevance in solar energy conversionarrow_forward
- Q2: Draw all applicable resonance forms for the acetate ion CH3COO. Clearly show all lone pairs, charges, and arrow formalism.arrow_forwardIndicate what metal clusters are.arrow_forward55. The photoelectric threshold energy for ytterbium metal is 4.16 × 10-19 J/atom. a. Calculate the wavelength of light that this energy corresponds to (in nm). b. Which region of the electromagnetic spectrum does this wavelength fall in? c. Would light of wavelength 490 nm produce a photoelectric effect in ytterbium? Why or why not?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY