
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.10P
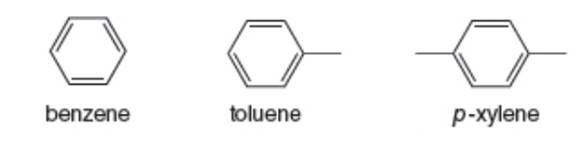
Benzene, toluene, and p-xylene (BTX) are often added to gasoline to boost octane ratings. What would be observed if a mixture of these three compounds were subjected to GC–MS analysis? How many peaks would be present in the gas chromatogram? What would be the relative order of the peaks? What molecular ions would be observed in the mass spectra?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
יווי
10
20
30
40
50
60
70
3.5
3
2.5
2
1.5
1
[ppm]
3.5
3
2.5
2
1.5
1
6 [ppm]
1
1.5
-2.5
3.5
2H2S(g)+3O2(g)→2SO2(g)+2H2O(g)
A 1.2mol sample of H2S(g) is combined with excess O2(g), and the reaction goes to completion.
Question
Which of the following predicts the theoretical yield of SO2(g) from the reaction?
Responses
1.2 g
Answer A: 1.2 grams
A
41 g
Answer B: 41 grams
B
77 g
Answer C: 77 grams
C
154 g
Answer D: 154 grams
D
Part VII. Below are the 'HNMR, 13 C-NMR, COSY 2D- NMR, and HSQC 2D-NMR (similar with HETCOR but axes are reversed) spectra of an
organic compound with molecular formula C6H1003 - Assign chemical shift values to the H and c atoms of the
compound. Find the structure. Show complete solutions.
Predicted 1H NMR Spectrum
4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1
f1 (ppm)
Predicted 13C NMR Spectrum
100
f1 (ppm)
30
220 210 200 190 180
170
160 150 140 130 120
110
90
80
70
-26
60
50
40
46
30
20
115
10
1.0 0.9 0.8
0
-10
Chapter 13 Solutions
Organic Chemistry-Package(Custom)
Ch. 13 - What is the mass of the molecular ion formed from...Ch. 13 - Prob. 13.2PCh. 13 - Prob. 13.3PCh. 13 - What molecular ions would you expect for the...Ch. 13 - The mass spectrum of 2,3-dimethylpentane also...Ch. 13 - The base peak in the mass spectrum of 2, 2,...Ch. 13 - (a) What mass spectral fragments are formed by ...Ch. 13 - What cations are formed in the mass spectrometer...Ch. 13 - The low-resolution mass spectrum of an unknown...Ch. 13 - Benzene, toluene, and p-xylene BTX are often added...
Ch. 13 - Prob. 13.11PCh. 13 - Prob. 13.12PCh. 13 - Prob. 13.13PCh. 13 - Prob. 13.14PCh. 13 - How do the IR spectra of the isomers cyclopentane...Ch. 13 - How do the three isomers of molecular formula...Ch. 13 - Problem 13.18 What functional groups are...Ch. 13 - Problem-13.19 What are the major IR absorptions in...Ch. 13 - Problem-13.20 What are the major IR absorptions in...Ch. 13 - Prob. 13.20PCh. 13 - Problem-13.22 Propose structures consistent with...Ch. 13 - 13.23 What major IR absorptions are present above ...Ch. 13 - Problem-13.24 The mass spectrum of the following...Ch. 13 - What molecular ion is expected for each compound?Ch. 13 - Which compound gives a molecular ion at m/z= 122,...Ch. 13 - Propose two molecular formulas for each molecular...Ch. 13 - Propose four possible structures for a hydrocarbon...Ch. 13 - Match each structure to its mass spectrum. a. b....Ch. 13 - 13.32 Propose two possible structures for a...Ch. 13 - 13.33 What cations are formed in the mass...Ch. 13 - 13.35 For each compound, assign likely...Ch. 13 - Prob. 13.32PCh. 13 - 13.37 Propose a structure consistent with each...Ch. 13 - 13.38 A low-resolution mass spectrum of the...Ch. 13 - Can the exact mass obtained in a high-resolution...Ch. 13 - 13.39 Primary alcohols often show a peak in their...Ch. 13 - 13.40 Like alcohols, ethers undergo α cleavage by...Ch. 13 - Which of the highlighted bonds absorbs at higher v...Ch. 13 - What major IR absorptions are present above...Ch. 13 - How would each of the following pairs of compounds...Ch. 13 - 13.44 Morphine, heroin, and oxycodone are three...Ch. 13 - Prob. 13.42PCh. 13 - 13.47 Match each compound to its IR spectrum
Ch. 13 - 13.48 Propose possible structures consistent with...Ch. 13 - A chiral hydrocarbon X exhibits a molecular ion at...Ch. 13 - 13.50 A chiral compound has a strong absorption...Ch. 13 - 13.51 Treatment of benzoic acid with followed by...Ch. 13 - 13.52 Treatment of benzaldehyde with in aqueous ...Ch. 13 - Prob. 13.49PCh. 13 - 13.54 Reaction of 2-methylpropanoic acid with ...Ch. 13 - 13.55 Reaction of pentanoyl chloride with lithium...Ch. 13 - Prob. 13.52PCh. 13 - 13.57 Treatment of anisole with and forms P,...Ch. 13 - 13.58 Reaction of with forms compound ,...Ch. 13 - Problem-13.59 The carbonyl absorption of an amide...Ch. 13 - Prob. 13.56PCh. 13 - Problem-13.61 Explain why a ketone carbonyl...Ch. 13 - 13.62 Oxidation of citronellol, a constituent of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forward4. Draw and label all possible isomers for [M(py)3(DMSO)2(CI)] (py = pyridine, DMSO dimethylsulfoxide).arrow_forwardThe emission data in cps displayed in Table 1 is reported to two decimal places by the chemist. However, the instrument output is shown in Table 2. Table 2. Iron emission from ICP-AES Sample Blank Standard Emission, cps 579.503252562 9308340.13122 Unknown Sample 343.232365741 Did the chemist make the correct choice in how they choose to display the data up in Table 1? Choose the best explanation from the choices below. No. Since the instrument calculates 12 digits for all values, they should all be kept and not truncated. Doing so would eliminate significant information. No. Since the instrument calculates 5 decimal places for the standard, all of the values should be limited to the same number. The other decimal places are not significant for the blank and unknown sample. Yes. The way Saman made the standards was limited by the 250-mL volumetric flask. This glassware can report values to 2 decimal places, and this establishes our number of significant figures. Yes. Instrumental data…arrow_forward
- 7. Draw a curved arrow mechanism for the following reaction. HO cat. HCI OH in dioxane with 4A molecular sievesarrow_forwardTry: Convert the given 3D perspective structure to Newman projection about C2 - C3 bond (C2 carbon in the front). Also, show Newman projection of other possible staggered conformers and circle the most stable conformation. Use the template shown. F H3C Br Harrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY