
Interpretation: The data for the dimerization of
Concept introduction: The change in the concentration of reactant or product at a particular instant of time is known as instantaneous
To determine: The instantaneous rate of change at
Answer to Problem 13.39QP
Solution
The instantaneous rates of change at
Explanation of Solution
Explanation
Given
The given data is,
|
Time
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 1
The graph for the above equation is obtained as,
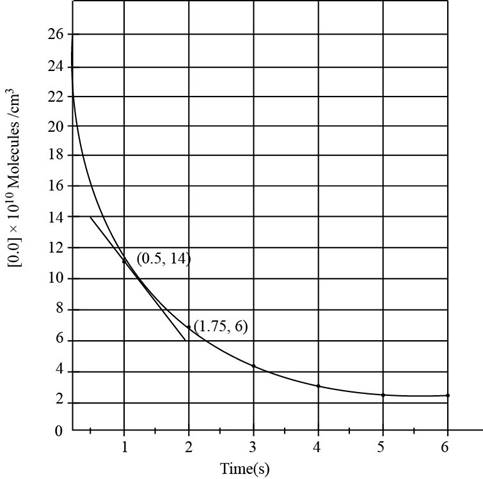
Figure 1
For finding out the instantaneous rate of change a tangent is drawn at
The change in the concentration of reactant between the given time period is given as,
Substitute the value of initial and final concentration of
The instantaneous reaction rate involving change in
The given reaction is,
Initially there was no
The value of
Substitute this value of
Substitute the value of final concentration and initial concentration of
Substitute the value of final concentration and initial concentration of
Substitute the value of final concentration and initial concentration of
Substitute the value of final concentration and initial concentration of
Substitute the value of final concentration and initial concentration of
Substitute the value of final concentration and initial concentration of
The obtained data is tabulated as,
|
Time
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 2
The graph from the above table is obtained as,
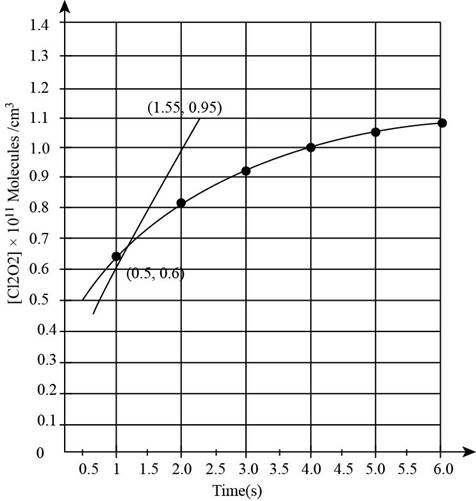
Figure 2
The change in the concentration of reactant between the given time period is given as,
Substitute the value of initial and final concentration of
The instantaneous reaction rate involving change in
Conclusion
The instantaneous rates of change at
Want to see more full solutions like this?
Chapter 13 Solutions
Chemistry: The Science in Context (Fifth Edition)
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





