
Concept explainers
(a)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates. For E2 elimination reactions, a strong base is used in the presence of a
Answer to Problem 13.33P
The solvent for the proposed synthetic step is appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). If the solvent ethanol is deprotonated, the result is still an ethoxide ion which is the same base that is already shown.
Explanation of Solution
The given proposed synthetic step is:
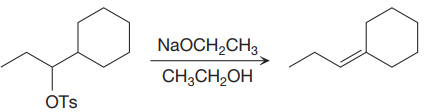
The reactant molecule has a good leaving group,
The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions).
(b)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates. For E2 elimination reactions, a strong base is used in the presence of a polar aprotic solvent to yield the most substituted alkene is the major product. The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions). If the base used in the reaction and the base produced by the deprotonation of solvent molecules is the same, then the reaction proceeds in the forward direction and the proposed synthetic step is valid and acceptable. If the base used in the reaction is different from the base produced by the deprotonation of solvent molecules, then they would compete and the proposed synthetic route then would not be the valid route.
Answer to Problem 13.33P
The solvent ethanol for the proposed synthetic step is not appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). The alkoxide ion shown
Explanation of Solution
The given proposed synthetic step is:
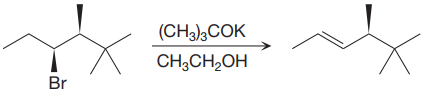
The reactant molecule has a good leaving group,
The requirement of the solvent for E2 reactions is aprotic, as aprotic solvents do not solvate the anions (negatively charged ions) as strongly as they solvate cations (positively charged ions).
(c)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates.
For a substitution reaction which follows
Answer to Problem 13.33P
The solvent ethanol for the proposed synthetic step is not appropriate. The proposed synthetic step is an example of a
Explanation of Solution
The given proposed synthetic step is:
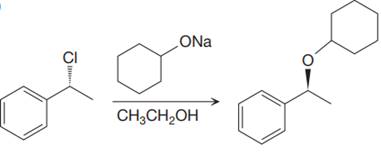
The reactant molecule has a moderate leaving group,
A
Thus, the solvent ethanol will interfere in the proposed synthetic step. Instead of ethanol cyclohexanol should be used, which will generate the same anion that is cyclohexanolate ion or any other aprotic solvent such as DMSO could be used.
The requirement of the solvent for
(d)
Interpretation:
It is to be determined whether the solvent would interfere with producing the intended target in the proposed synthetic step. For those that would, another solvent that can be used is to be suggested with an explanation.
Concept introduction:
Solvent plays an important role in the proposed synthetic step by not interfering with the reactant molecule or intermediates.
For a substitution reaction which follows
Answer to Problem 13.33P
The solvent for the proposed synthetic step is appropriate. The proposed synthetic step is an example of an E2 reaction, which favors the Zaitsev product (most substituted alkene). If the solvent ethanol is deprotonated, the result is still an ethoxide ion which is the same base that is already shown.
Explanation of Solution
The given proposed synthetic step is:
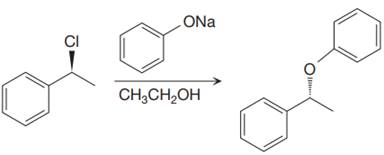
The reactant molecule has a good leaving group,
The requirement of the solvent for
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forward
- Can I please get help with this? And can I please the lowest possible significant number?arrow_forwardWhat is the molar mass of a gas that takes three times longer to effuse than helium?arrow_forwardFirst image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forward
- what is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forwardOne step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
