
ORG CHEM CONNECT CARD
6th Edition
ISBN: 9781264860746
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12C.9, Problem 23P
What protons in alcohol A give rise to each signal in its
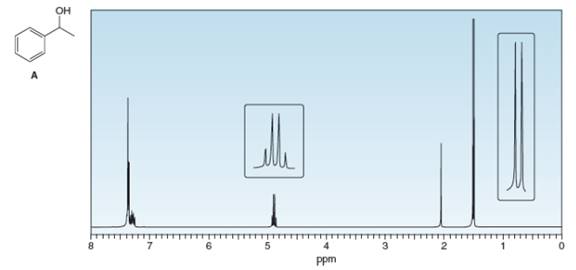
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The chemical reaction you investigated is a two-step reaction. What type of reaction occurs in each step? How did you determine your answer?
What is the relationship between the limiting reactant and theoretical yield of CO2?
From your calculations, which reaction experiment had closest to stoichiometric quantities? How many moles of NaHCO3 and HC2H3O2 were present in this reaction?
Chapter 12C Solutions
ORG CHEM CONNECT CARD
Ch. 12C.1 - Problem 14.1 The NMR spectrum of recorded on a ...Ch. 12C.1 - Prob. 2PCh. 12C.2 - How many 1H NMR signals does each...Ch. 12C.2 - How many 1H NMR signals does each compound give?...Ch. 12C.2 - Label the protons in each highlighted CH2 group as...Ch. 12C.2 - How many 1H NMR signals would you expect for each...Ch. 12C.3 - Prob. 9PCh. 12C.3 - For each compound, first label each different type...Ch. 12C.3 - Label each statement as True or False. a. When a...Ch. 12C.4 - Prob. 12P
Ch. 12C.5 - Problem 14.12 Which compound give a NMR spectrum...Ch. 12C.5 - Prob. 14PCh. 12C.6 - Prob. 15PCh. 12C.6 - For each compound give the number of 1H NMR...Ch. 12C.6 - Prob. 17PCh. 12C.7 - Prob. 18PCh. 12C.7 - Problem 14.18 Describe the NMR spectrum of each...Ch. 12C.8 - Problem 14.19 Draw a splitting diagram for in ,...Ch. 12C.8 - Problem 14.20 Identify A and B, isomers of...Ch. 12C.9 - Problem 14.21 How many signals are present in the ...Ch. 12C.9 - Problem 14.22 What protons in alcohol A give rise...Ch. 12C.9 - How many peaks are observed in the NMR signal for...Ch. 12C.10 -
Problem 14.24 Propose a structure for a compound...Ch. 12C - 14.34 (a) How many NMR signals does each of the...Ch. 12C - 14.35 (a) How many NMR signals does each compound...Ch. 12C - Prob. 38PCh. 12C - 14.37 How many NMR signals does each natural...Ch. 12C - Prob. 40PCh. 12C - 14.39 What effect does increasing the operating...Ch. 12C - Prob. 43PCh. 12C - Prob. 44PCh. 12C - Prob. 45PCh. 12C - Prob. 47PCh. 12C - Prob. 48PCh. 12C - 14.48 How many NMR signals does each compound...Ch. 12C - 14.49 Rank the highlighted carbon atoms in each...Ch. 12C - 14.50 Identify the carbon atoms that give rise to...Ch. 12C - 14.62 Reaction of with , followed by treatment...Ch. 12C - Reaction of aldehyde D with amino alcohol E in the...Ch. 12C - 14.64 Propose a structure consistent with each set...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Give the IUPAC name for each compound.
Organic Chemistry
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
What are the cervical and lumbar enlargements?
Principles of Anatomy and Physiology
1. Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
the interactio...
Biochemistry: Concepts and Connections (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 18. Arrange the following carbocations in order of decreasing stability. 1 2 A 3124 B 4213 C 2431 D 1234 E 2134 SPL 3 4arrow_forwardAcetic acid is added to DI water at an initial concentration of 10 -6 M (Ka=1.8x10-5) A. Using the "ICE" Method, what would the pH be at equilibrium? State assumptions and show your work. B. Using the simultaneous equations method, what would the pH be at equilibrium? Show your workarrow_forward1. Show that the change in entropy for a fixed amount of ideal gas held at a constant temperature undergoing a volume change is given by the simple equation AS = NkB In Hint: Start with the equation M dS = du + (Œ) dv - Ž (#) an, dU du+av-dN; j=1 Why doesn't the equation for the entropy of an ideal gas depend on the strength of the intermolecular forces for the gas?arrow_forward
- 2. Make an ice cube at 1 bar pressure by freezing an amount of liquid water that is 2 cm x 2 cm x 2 cm in volume. The density of liquid water at 0 °C is 1.000 g cm³ and the density of ice at 0 °C is 0.915 g cm³. Note that this difference in density is the reason your water pipes burst if they freeze and why you shouldn't forget to take your bottle of pop out of the freezer if you put it in there to try and cool it down faster. A. What is the work of expansion upon freezing? B. Is work done on the system or by the system?arrow_forwardI have a excitation/emission spectra of a quinine standard solution here, and I'm having trouble interpreting it. the red line is emission the blue line is excitation. i'm having trouble interpreting properly. just want to know if there is any evidence of raman or rayleigh peaks in the spectra.arrow_forwardGive the major product of the following reaction. excess 1. OH, H₂O 1.OH H CH3CH2CH21 H 2. A.-H₂O Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default.arrow_forward
- 2. Use Hess's law to calculate the AH (in kJ) for: rxn CIF(g) + F2(g) → CIF 3 (1) using the following information: 2CIF(g) + O2(g) → Cl₂O(g) + OF 2(g) AH = 167.5 kJ ΔΗ 2F2 (g) + O2(g) → 2 OF 2(g) 2C1F3 (1) + 202(g) → Cl₂O(g) + 3 OF 2(g) о = = -43.5 kJ AH = 394.1kJarrow_forwardci Draw the major product(s) of the following reactions: (3 pts) CH3 HNO3/H2SO4 HNO3/ H2SO4 OCH3 (1 pts)arrow_forwardProvide the product for the reactionarrow_forward
- What is the net ionic equation for the reaction between tin(IV) sulfide and nitric acid?arrow_forwardThe combustion of 28.8 g of NH3 consumes exactly _____ g of O2. 4 NH3 + 7 O2 ----> 4 NO2 + 6 H2Oarrow_forwardWhat is the molecular formula of the bond-line structure shown below OH HO ○ C14H12O2 ○ C16H14O2 ○ C16H12O2 O C14H14O2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY