
EBK ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260475685
Author: SMITH
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12C.2, Problem 6P
How many
a. 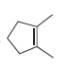 b.
b. 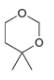 c.
c. 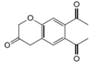 d.
d. 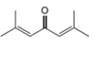
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2.
Add the following group of numbers using the correct number of significant figures for the
answer. Show work to earn full credit such as rounding off the answer to the correct number
of significant figures. Replace the question marks with the calculated answers or write
the calculated answers near the question marks.
10916.345
37.40832
5.4043
3.94
+
0.0426
?
(7 significant figures)
The emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.
Indicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.
Chapter 12C Solutions
EBK ORGANIC CHEMISTRY
Ch. 12C.1 - Problem 14.1 The NMR spectrum of recorded on a ...Ch. 12C.1 - Prob. 2PCh. 12C.2 - How many 1H NMR signals does each...Ch. 12C.2 - How many 1H NMR signals does each compound give?...Ch. 12C.2 - Label the protons in each highlighted CH2 group as...Ch. 12C.2 - How many 1H NMR signals would you expect for each...Ch. 12C.3 - Prob. 9PCh. 12C.3 - For each compound, first label each different type...Ch. 12C.3 - Label each statement as True or False. a. When a...Ch. 12C.4 - Prob. 12P
Ch. 12C.5 - Problem 14.12 Which compound give a NMR spectrum...Ch. 12C.5 - Prob. 14PCh. 12C.6 - Prob. 15PCh. 12C.6 - For each compound give the number of 1H NMR...Ch. 12C.6 - Prob. 17PCh. 12C.7 - Prob. 18PCh. 12C.7 - Problem 14.18 Describe the NMR spectrum of each...Ch. 12C.8 - Problem 14.19 Draw a splitting diagram for in ,...Ch. 12C.8 - Problem 14.20 Identify A and B, isomers of...Ch. 12C.9 - Problem 14.21 How many signals are present in the ...Ch. 12C.9 - Problem 14.22 What protons in alcohol A give rise...Ch. 12C.9 - How many peaks are observed in the NMR signal for...Ch. 12C.10 -
Problem 14.24 Propose a structure for a compound...Ch. 12C - 14.34 (a) How many NMR signals does each of the...Ch. 12C - 14.35 (a) How many NMR signals does each compound...Ch. 12C - Prob. 38PCh. 12C - 14.37 How many NMR signals does each natural...Ch. 12C - Prob. 40PCh. 12C - 14.39 What effect does increasing the operating...Ch. 12C - Prob. 43PCh. 12C - Prob. 44PCh. 12C - Prob. 45PCh. 12C - Prob. 47PCh. 12C - Prob. 48PCh. 12C - 14.48 How many NMR signals does each compound...Ch. 12C - 14.49 Rank the highlighted carbon atoms in each...Ch. 12C - 14.50 Identify the carbon atoms that give rise to...Ch. 12C - 14.62 Reaction of with , followed by treatment...Ch. 12C - Reaction of aldehyde D with amino alcohol E in the...Ch. 12C - 14.64 Propose a structure consistent with each set...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
the interactio...
Biochemistry: Concepts and Connections (2nd Edition)
Give the IUPAC name for each compound.
Organic Chemistry
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forwardUsing Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forward
- The molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forwardBA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward
- 4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a) NHBoc ⚫OBn HO. H3C CO2CH3 -OBn H3C H3C. H3C. NHBOC CI CO2CH3arrow_forwardDraw structures of the following compounds and identify their role: mCPBA (MCPBA) DMS Py 9-BBN LAH Sia₂BH TsCI PCC t-BuOK LDA MeLi n-BuLi DMSO DMF Sodium Borohydride Lithium DiisopropylAmide 2arrow_forwardUsing Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode. DATA: Electrode potentials E° = 0,854 V y E 0,788 V Hg2+/Hg 2+ Hg2/Hgarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY