
Concept explainers
Interpretation:
The steps how the two products formed from reaction of methylenecyclohehane with NBS has to be given. Stereoisomers has to be disregarded.
Concept introduction:
Bromination of Allylic Carbons:
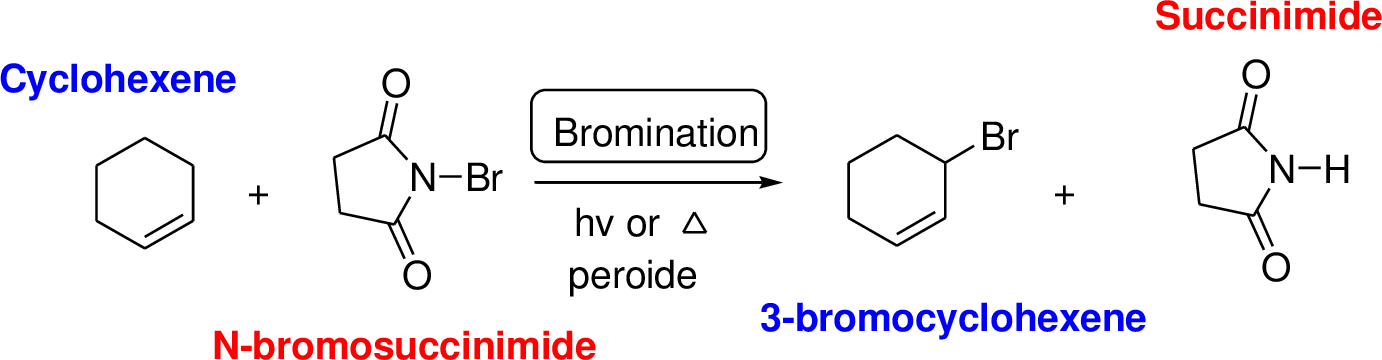
N-bromosuccinimide (NBS) is used for the allylic bromination through radical reaction. Bromination of allylicc carbon requires low concentration of bromine and low concentration of hydrobromic acid. If high concentration of bromine and high concentration of hydrobromic acid which leads to the formation of bromonation in the double bond.
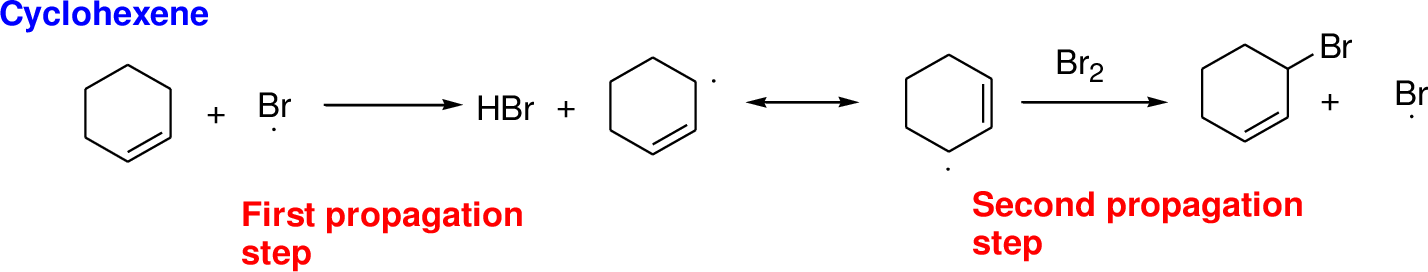
Bromination reaction starts with the homolytic cleavage of N-Br bond in N-bromosuccinimide (NBS) which creates bromine radical to initiate the radical bromination reaction.
NBS bromine radical removes the allylic hydrogen which forms hydrogen bromide and allylic radical in the first propagation step, the allylic radical is stabilized by the double bond in ring. This allylic radical reaction with bromine molecule and forms allylic bromide in the second propagation step which are shown above.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry (8th Edition)
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward
