
GENERAL,ORGANIC,+BIOCHEMISTRY
10th Edition
ISBN: 9781260148954
Author: Denniston
Publisher: RENT MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 12.2, Problem 12.2PP
(a)
Interpretation Introduction
Interpretation:
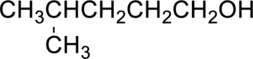
Concept Introduction:
A common nomenclature of naming organic compounds has been developed by IUPAC. By usage of this nomenclature or rules, memorizing of names of organic compounds is not necessary.
IUPAC rules for naming alcohols:
- Alcohols are named by identifying the parent compound and replacing the –e ending with –ol.
- Lowest possible number should be given for the hydroxyl group present in the parent chain.
- Name and number all substituent, and put them as prefixes to “alkanol” name also give the first preference as per the alphabetical order.
- If an alcohol contains two hydroxyl groups then name it as
diol , an alcohol; contains three hydroxyl groups then name it as triol. - Common names for alcohols are derived from alkyl group corresponding to the parent compound.
(b)
Interpretation Introduction
Interpretation:
IUPAC nomenclature for the given compound has to be given.
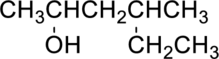
Concept Introduction:
Refer part (a).
(c)
Interpretation Introduction
Interpretation:
IUPAC nomenclature for the given compound glycerol has to be given.
Concept Introduction:
Refer part (a).
(d)
Interpretation Introduction
Interpretation:
IUPAC nomenclature for the given compound has to be given.
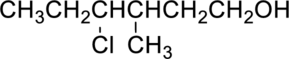
Concept Introduction:
Refer part (a).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.
Question 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction
Please draw all structures clearly. Note that this intramolecular cyclization is analogous
to the mechanism for halohydrin formation.
COH
Br
+ HBr
Br
Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.
Chapter 12 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY
Ch. 12.1 - Prob. 12.1PPCh. 12.2 - Prob. 12.2PPCh. 12.2 - Prob. 12.3PPCh. 12.2 - Prob. 12.1QCh. 12.2 - Prob. 12.2QCh. 12.4 - Write an equation representing the reduction of...Ch. 12.4 - Write an equation representing the reduction of...Ch. 12.4 - Prob. 12.6PPCh. 12.4 - Prob. 12.7PPCh. 12.4 - Prob. 12.8PP
Ch. 12.4 - Prob. 12.3QCh. 12.4 - Prob. 12.4QCh. 12.4 - Prob. 12.5QCh. 12.4 - Prob. 12.6QCh. 12.4 - Prob. 12.7QCh. 12.4 - Prob. 12.8QCh. 12.6 - Prob. 12.9QCh. 12.6 - Prob. 12.10QCh. 12.7 - Name the following ethers using IUPAC...Ch. 12.7 - Prob. 12.10PPCh. 12.7 - Prob. 12.11PPCh. 12.7 - Prob. 12.11QCh. 12.7 - Prob. 12.12QCh. 12.8 - Prob. 12.12PPCh. 12 - Prob. 12.13QPCh. 12 - Prob. 12.14QPCh. 12 - Prob. 12.15QPCh. 12 - Prob. 12.16QPCh. 12 - Prob. 12.17QPCh. 12 - Prob. 12.18QPCh. 12 - Prob. 12.19QPCh. 12 - Prob. 12.20QPCh. 12 - Stingless bees use complex systems to communicate....Ch. 12 - Prob. 12.22QPCh. 12 - Prob. 12.23QPCh. 12 - Prob. 12.24QPCh. 12 - Prob. 12.25QPCh. 12 - Prob. 12.26QPCh. 12 - Prob. 12.27QPCh. 12 - Prob. 12.28QPCh. 12 - Prob. 12.29QPCh. 12 - Prob. 12.30QPCh. 12 - Prob. 12.31QPCh. 12 - Prob. 12.32QPCh. 12 - Prob. 12.33QPCh. 12 - Prob. 12.34QPCh. 12 - Prob. 12.35QPCh. 12 - Prob. 12.36QPCh. 12 - Prob. 12.37QPCh. 12 - Prob. 12.38QPCh. 12 - Prob. 12.39QPCh. 12 - Prob. 12.40QPCh. 12 - Prob. 12.41QPCh. 12 - Prob. 12.42QPCh. 12 - Prob. 12.43QPCh. 12 - Prob. 12.44QPCh. 12 - Prob. 12.45QPCh. 12 - Prob. 12.46QPCh. 12 - Prob. 12.47QPCh. 12 - Prob. 12.48QPCh. 12 - Prob. 12.49QPCh. 12 - Prob. 12.50QPCh. 12 - Prob. 12.51QPCh. 12 - Prob. 12.52QPCh. 12 - Prob. 12.53QPCh. 12 - Prob. 12.54QPCh. 12 - Prob. 12.55QPCh. 12 - Prob. 12.56QPCh. 12 - Prob. 12.57QPCh. 12 - Prob. 12.58QPCh. 12 - Prob. 12.59QPCh. 12 - Prob. 12.60QPCh. 12 - Prob. 12.61QPCh. 12 - Prob. 12.62QPCh. 12 - Prob. 12.63QPCh. 12 - Prob. 12.64QPCh. 12 - Prob. 12.65QPCh. 12 - Prob. 12.66QPCh. 12 - Prob. 12.67QPCh. 12 - Prob. 12.68QPCh. 12 - Prob. 12.69QPCh. 12 - Prob. 12.70QPCh. 12 - Prob. 12.71QPCh. 12 - Prob. 12.72QPCh. 12 - Prob. 12.73QPCh. 12 - Prob. 12.74QPCh. 12 - Prob. 12.75QPCh. 12 - Prob. 12.76QPCh. 12 - Prob. 12.77QPCh. 12 - Prob. 12.78QPCh. 12 - Prob. 12.79QPCh. 12 - Prob. 12.80QPCh. 12 - Prob. 12.81QPCh. 12 - Prob. 12.82QPCh. 12 - Prob. 12.83QPCh. 12 - Prob. 12.84QPCh. 12 - Prob. 12.85QPCh. 12 - Write the IUPAC and common name for each of the...Ch. 12 - Prob. 12.87QPCh. 12 - Prob. 12.88QPCh. 12 - Prob. 12.89QPCh. 12 - Prob. 12.90QPCh. 12 - Prob. 12.91QPCh. 12 - Prob. 12.92QPCh. 12 - Prob. 1MCPCh. 12 - Prob. 2MCPCh. 12 - Prob. 3MCPCh. 12 - Prob. 4MCPCh. 12 - Prob. 10MCPCh. 12 - Prob. 11MCP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forwardAdd conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward
- Steps on how to solve. Thank you!arrow_forward3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward1. Which one(s) of these can be oxidized with CrO3 ? (could be more than one) a) triphenylmethanol b) 2-pentanol c) Ethyl alcohol d) CH3 2. Write in all the product(s) of this reaction. Label them as "major" or "minor". 2-methyl-2-hexanol H2SO4, heatarrow_forward
- 3) Determine if the pairs are constitutional isomers, enantiomers, diastereomers, or mesocompounds. (4 points)arrow_forwardIn the decomposition reaction in solution B → C, only species C absorbs UV radiation, but neither B nor the solvent absorbs. If we call At the absorbance measured at any time, A0 the absorbance at the beginning of the reaction, and A∞ the absorbance at the end of the reaction, which of the expressions is valid? We assume that Beer's law is fulfilled.arrow_forward> You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: 1. ☑ CI 2. H3O+ O Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. Explanation Check ? DO 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY