
Chemistry Atoms First2e
2nd Edition
ISBN: 9781947172647
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 9E
Consider a system similar to the one in Figure 128, except that it contains six particles instead of four. What is the probability of having all the particles in only one of the two boxes in the case? Compare this with the similar probability for the system of four particles that we have derived to be equal to
What does this comparison tell us about even larger systems? 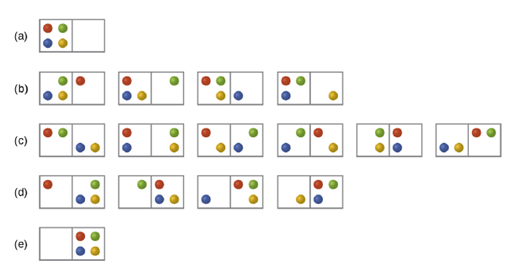
Figure 12.8 The sixteen microstates associated with placing four particles in two boxes are shown. The microstates are collected into five distributions-(a), (b), (c), (d), and (e)-based on the numbers of particles in each box.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
12. Mark the correct statement about
reactions a and b :
a.
Br
+ -OH
Br
b.
+ Br
H₂O
+
Br
-OH
+
H₂O
A) The reactions are elimination
reactions, with reaction "a" being of type
E2 and reaction "b" being of type E1.
B) Reaction "a" is an E2 type elimination
occurring in one step and reaction "b" is
an SN1 type substitution.
C) Both reactions can result in the
formation of carbocation, but in reaction
"b" the most stable carbocation will be
formed.
D) Both reactions occur at the same rate
○ and have the same number of reaction
steps.
E) Reaction "b" is an E2 type elimination
occurring in two steps and reaction "a" is
an SN2 type substitution.
Chloroform, long used as an anesthetic and now considered carcinogenic, has a heat of vaporization of 31.4 kJ/mol. During vaporization, its entropy increases by 94.2 J/mol.K. Therefore, select the alternative that indicates the temperature, in degrees Celsius, at which chloroform begins to boil under a pressure of 1 atm.
A) 28
B) 40
C) 52
D) 60
E) 72
If we assume a system with an anodic overpotential, the variation of n as a function
of current density:
1. at low fields is linear 2. at higher fields, it follows Tafel's law
Obtain the range of current densities for which the overpotential has the same value
when calculated for 1 and 2 cases (maximum relative difference of 5% compared to
the behavior for higher fields).
To which overpotential range does this correspond?
Data: i = 1.5 mA cm², T = 300°C, B = 0.64, R = 8.314 J K1 mol-1 and F = 96485 C mol-1.
Chapter 12 Solutions
Chemistry Atoms First2e
Ch. 12 - What is a spontaneous reaction?Ch. 12 - What is a nonspontaneous reaction?Ch. 12 - Indicate whether the following processes are...Ch. 12 - A helium-filled balloon spontaneously deflates...Ch. 12 - Many plastic materials are organic polymers that...Ch. 12 - In Figure 12.8 all possible distributions and...Ch. 12 - In Figure 12.8 all possible distributions and...Ch. 12 - How does the process described in the previous...Ch. 12 - Consider a system similar to the one in Figure...Ch. 12 - Consider the system shown in Figure 12.9. What is...
Ch. 12 - Consider the system shown in Figure 12.9. What is...Ch. 12 - Arrange the following sets of systems in order of...Ch. 12 - At room temperature, the entropy of the halogens...Ch. 12 - Consider two processes: sublimation of I2(s) and...Ch. 12 - Indicate which substance in the given pairs has...Ch. 12 - Predict the sign of the entropy change for the...Ch. 12 - Predict the sign of the entropy change for the...Ch. 12 - Write the balanced chemical equation for the...Ch. 12 - Write the balanced chemical equation for the...Ch. 12 - What is the difference between S and S for a...Ch. 12 - Calculate S for the following changes. (a)...Ch. 12 - Determine the entropy change for the combustion of...Ch. 12 - Determine the entropy change for the combustion of...Ch. 12 - Thermite reactions have been used for welding...Ch. 12 - Using the relevant S values listed in Appendix G,...Ch. 12 - From the following information, determine S for...Ch. 12 - By calculating Suniv, at each temperature,...Ch. 12 - Use the standard entropy data in Appendix G to...Ch. 12 - Use the standard entropy data in Appendix G to...Ch. 12 - What is the difference between G and G for a...Ch. 12 - A reaction has H=100kJ/mol and S=250J/mol.K . Is...Ch. 12 - Explain what happens as a reaction starts with G0...Ch. 12 - Use the standard free energy of formation data in...Ch. 12 - Use the standard free energy data in Appendix G to...Ch. 12 - Given: P4(s)+5O2(g)P4O10(s) G=2697.0kJ/mol...Ch. 12 - Is the formation of ozone (O3(g)) from oxygen...Ch. 12 - Consider the decomposition of red mercury(II)...Ch. 12 - Among other things, an ideal fuel for the control...Ch. 12 - Calculate G for each of the following reactions...Ch. 12 - Calculate G for each of the following reactions...Ch. 12 - Calculate the equilibrium constant at 25 C for...Ch. 12 - Determine G for the following reactions. (a)...Ch. 12 - Given that the Gf for Pb2+(aq) and Cl-(aq) is...Ch. 12 - Determine the standard free energy change, Gf, for...Ch. 12 - Determine the standard enthalpy change, entropy...Ch. 12 - The evaporation of one mole of water at 298 K has...Ch. 12 - In glycolysis, the reaction of glucose (Glu) to...Ch. 12 - One of the important reactions in the biochemical...Ch. 12 - Without doing a numerical calculation, determine...Ch. 12 - When ammonium chloride is added to water and...Ch. 12 - An important source of copper is from the copper...Ch. 12 - What happens to G (becomes more negative or more...
Knowledge Booster
Similar questions
- Answer by equation pleasearrow_forwardSome of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.arrow_forwardWhen talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forward
- Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forwardWhat is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forward
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning