
Concept explainers
Write a balanced equation for the combustion of each sycloalkane.
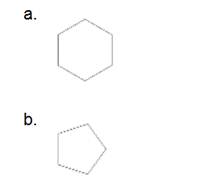
(a)
Interpretation:
The balance equation for the combustion of following cycloalkane should be written.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and their atoms doesn't form a ring is known as acyclic compounds.
The chemical reaction which involves the reaction of alkane and oxygen, results in the formation of carbon dioxide gas and water vapor is known as combustion reaction. The combustion reaction is an exothermic reaction.
The general reaction of combustion of alkane is:
A reaction is said to be balanced, if the number of atoms of each element on the product side and one the reactant side of a chemical reaction are equal.
Answer to Problem 66P
Explanation of Solution
Cyclic alkane is defined as an alkane in which series of atoms are combined to form a ring.
The general molecular formula of cyclic alkane is
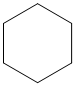
The given cycloalkane is:
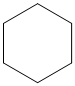
The chemical formula of the cycloalkane is
The combustion reaction of
Reactant side Product side
Number of C atoms = 6 Number of C atoms = 1
Number of H atoms = 12 Number of H atoms = 2
Number of O atoms = 2 Number of O atoms = 3
Thus, the above reaction is not balanced. To balance the reaction, multiply 9 with oxygen gas, 6 with carbon dioxide gas and 6 with water vapor.
Therefore, the balanced combustion reaction of
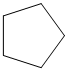
(b)
Interpretation:
The balance equation for the combustion of following cycloalkane should be written.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and their atoms doesn't form a ring is known as acyclic compounds.
The chemical reaction which involves the reaction of alkane and oxygen, results in the formation of carbon dioxide gas and water vapor is known as combustion reaction. The combustion reaction is an exothermic reaction.
The general reaction of combustion of alkane is:
A reaction is said to be balanced, if the number of atoms of each element on the product side and one the reactant side of a chemical reaction are equal.
Answer to Problem 66P
Explanation of Solution
Cyclic alkane is defined as an alkane in which series of atoms are combined to form a ring.
The general molecular formula of cyclic alkane is
The given cycloalkane is:
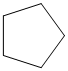
The chemical formula of the cycloalkane is
The combustion reaction of
Reactant side Product side
Number of C atoms = 5 Number of C atoms = 1
Number of H atoms = 10 Number of H atoms = 2
Number of O atoms = 2 Number of O atoms = 3
Thus, the above reaction is not balanced. To balance the reaction, multiply
Therefore, the balanced combustion reaction of
Want to see more full solutions like this?
Chapter 12 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forward
- Briefly state the electrocapillary equation for ideally polarized electrodes.arrow_forwardWhat is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forward
- The molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





