
Which substances will be soluble in water? (a) sodium chloride, (b) sugar, (c) calcium carbonate, (d) methanol, (c) biphenyl:
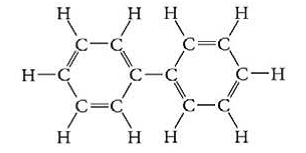
(Hint: What makes a substance soluble in water?)
Interpretation:
The substances which are soluble in water out of the given substances are to be identified.
Concept Introduction:
The force of attraction between the solvent molecules and between the solute molecules to form a liquid solution must be strong. When solute and solvent molecules have the same polarity, they form a solution and, when solute and solvent molecules have different polarity, they do not form a solution.
This principal also applies to the solubility of solid and liquid solvents. The solid and liquid molecules with same polarity combine to form a solution.
Answer to Problem 1PE
Correct answer: Options (a), (b), and (d)
Explanation of Solution
Reason for correct options:
Sodium chloride has ions or can be broken into ions, and water is also a polar solvent. So, according to the like dissolves like solubility rule, sodium chloride is soluble in water.
Sugars are polar molecules and can be broken into ions, and water is also a polar solvent. So, according to the like dissolves like solubility rule, sugar is soluble in water.
Methanol is an alcohol and has polar groups that can be broken into ions, and water is also a polar solvent. So, according to the like dissolves like solubility rule, methanol is soluble in water.
Hence, options (a), (b), and (d) are correct.
Reason for incorrect options:
Calcium carbonate given in option (c) is an ionic compound. Thus, it is polar but is insoluble in water because of its very high lattice energy. So, calcium carbonate is insoluble in water.
Biphenyl given in option (e) is a non-polar compound. As from the structure, it is clear that it has no polar groups, no charges, hence no dipole. Thus, according to the like dissolves like solubility rule, it is does not dissolve in water as water is a polar solvent.
Hence, options (c) and (e) are incorrect.
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry: The Molecular Nature of Matter
Additional Science Textbook Solutions
Brock Biology of Microorganisms (15th Edition)
Organic Chemistry (8th Edition)
Microbiology: An Introduction
Introductory Chemistry (6th Edition)
Chemistry: A Molecular Approach (4th Edition)
Chemistry: The Central Science (14th Edition)
- 5. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn. H3C CH3arrow_forwardState the name and condensed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardState the name and condensed formula of the isothiazole obtained by reacting acetylacetone and thiosemicarbazide.arrow_forward
- Provide the semi-developed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardGiven a 1,3-dicarbonyl compound (R1-CO-CH2-CO-R2), indicate the formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardAn orange laser has a wavelength of 610 nm. What is the energy of this light?arrow_forward
- The molar absorptivity of a protein in water at 280 nm can be estimated within ~5-10% from its content of the amino acids tyrosine and tryptophan and from the number of disulfide linkages (R-S-S-R) between cysteine residues: Ε280 nm (M-1 cm-1) ≈ 5500 nTrp + 1490 nTyr + 125 nS-S where nTrp is the number of tryptophans, nTyr is the number of tyrosines, and nS-S is the number of disulfide linkages. The protein human serum transferrin has 678 amino acids including 8 tryptophans, 26 tyrosines, and 19 disulfide linkages. The molecular mass of the most dominant for is 79550. Predict the molar absorptivity of transferrin. Predict the absorbance of a solution that’s 1.000 g/L transferrin in a 1.000-cm-pathlength cuvet. Estimate the g/L of a transferrin solution with an absorbance of 1.50 at 280 nm.arrow_forwardIn GC, what order will the following molecules elute from the column? CH3OCH3, CH3CH2OH, C3H8, C4H10arrow_forwardBeer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forward
- How to calculate % of unknown solution using line of best fit y=0.1227x + 0.0292 (y=2.244)arrow_forwardGiven a 1,3-dicarbonyl compound, state the (condensed) formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardComplete the following acid-base reactions and predict the direction of equilibrium for each. Justify your prediction by citing pK values for the acid and conjugate acid in each equilibrium. (a) (b) NHs (c) O₂N NH NH OH H₁PO₁arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





