
Concept explainers
Explain why each name is incorrect and then write a correct name. Strategy: First draw the structural formula suggested by the incorrect name. Then identify the error, for example, failure to locate the longest chain that contains the
(a) 1-Methylpropene (b) 3-Pentene
(c) 2-Methylcyclohexene (d) 3,3-Dimethylpentene
(e) 4-Hexyne
(f) 2-lsopropyl-2-butene
(a)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
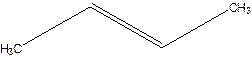
But-2-ene.
Explanation of Solution
1-Methylpropene.
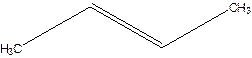
Error- longest chain not correctly located.
Correct name-
But-2-ene.
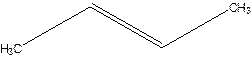
But-2-ene.
(b)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
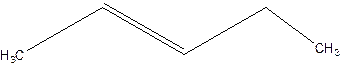
2-pentene.
Explanation of Solution
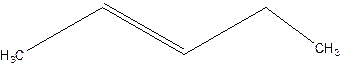
3-Pentene.
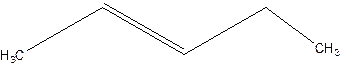
Error- Position of double bond not mentioned correctly.
Correct name-
2-Pentene.
(c)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
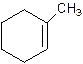
1-Methylcyclo-1-hexene.
Explanation of Solution
2-Methylcyclohexene.
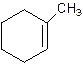
Error- Position of methyl not correct.
Correct name-
1-Methylcyclo-1-hexene.
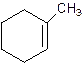
(d)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
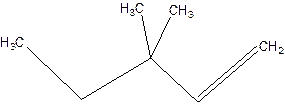
3,3-Dimethyl-1-pentene.
Explanation of Solution
3,3-Dimethylpentene.
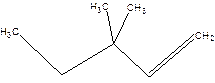
Error-position of double bond not mentioned.
Correct name-
3,3-Dimethyl-1-pentene.
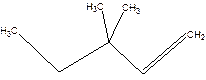
(e)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
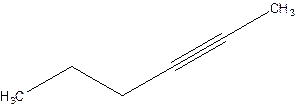
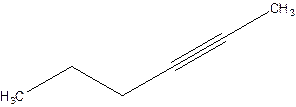
2-Hexyne.
Explanation of Solution
4-Hexyne.
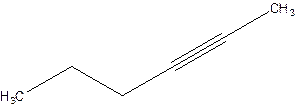
Error- position of triple bond is incorrectly mentioned.
Correct name 2-Hexyne.
(f)
Interpretation:
To analyse the error and write the correct IUPAC names.
Concept Introduction:
The rules to write the IUPAC names are as follows-
- The longest carbon chain is identified, and the root name is given accordingly.
- All the substituents attached to the root chain are determined.
- The position of the substituents is so assigned that the sum of their positions comes to be the least of all possible positions.
- The prefix di, tri, tetra is used if the same substituent is present two, three and four times respectively.
- All the substituents in the name are written in alphabetical order.
- For a cyclic hydrocarbon the prefix cyclo- is used.
Answer to Problem 16P
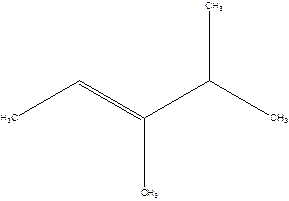
3,4-Dimethyl-2-pentene.
Explanation of Solution
2-Isopropyl-2-butene.
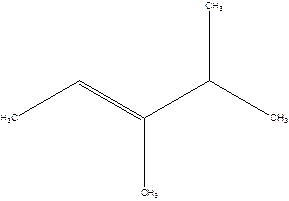
Error- longest chain is not correctly located.
The correct name will be-
3,4-Dimethyl-2-pentene.
Want to see more full solutions like this?
Chapter 12 Solutions
Introduction to General, Organic and Biochemistry
- curved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forwardUsing the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forwardSynthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forward
- Shown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forwardHi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forward
- Draw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M HCl is titrated with 37.75 mL of NaOH. What is the molarity of the NaOH?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





