
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
4th Edition
ISBN: 9781119761068
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 1.2, Problem 1.2P
Interpretation Introduction
Interpretation:
A systematic name for the given compound has to be provided.
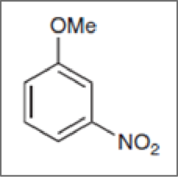
Concept Introduction:
Nomenclature of
- The parent is benzene ring.
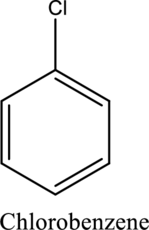
Functional groups attached to it other than hydrogens are called substituents. The name of substituent must be placed before the name of the compound as a prefix in any substituted hydrocarbon.Ex.
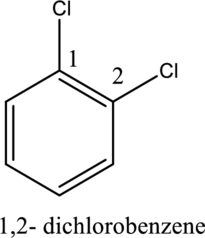
- The numerical prefixes such as di, tri, and tetra must be included in the nomenclature if more than one similar substituents are attached to the benzene ring.
Ex.
- If different substituent groups are attached to the benzene ring, the no. one position is determined by the parent. So the first step is to choose a suitable parent. Then the locants are assigned in a manner that gives the lower possible number to the next substituent.
Ex.
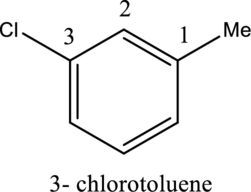
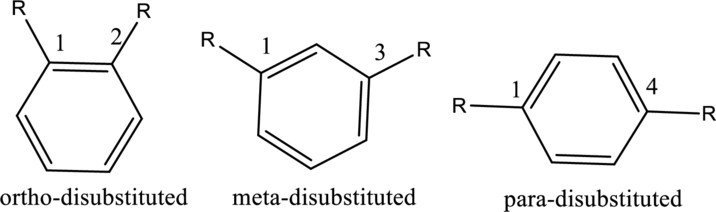
- For disubstituted benzenes, prefixes such as ortho, meta and para should be used.
Ex.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
16. The proton NMR spectral information shown in this problem is for a compound with formula
CioH,N. Expansions are shown for the region from 8.7 to 7.0 ppm. The normal carbon-13 spec-
tral results, including DEPT-135 and DEPT-90 results, are tabulated:
7
J
Normal Carbon
DEPT-135
DEPT-90
19 ppm
Positive
No peak
122
Positive
Positive
cus
и
124
Positive
Positive
126
Positive
Positive
128
No peak
No peak
4°
129
Positive
Positive
130
Positive
Positive
(144
No peak
No peak
148
No peak
No peak
150
Positive
Positive
してし
3. Propose a synthesis for the following transformation. Do not draw an arrow-pushing
mechanism below, but make sure to draw the product of each proposed step (3 points).
+ En
CN
CN
Show work..don't give Ai generated solution...
Chapter 1 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
Ch. 1.2 - Prob. 1.2PCh. 1.2 - Prob. 1.3PCh. 1.2 - Prob. 1.4PCh. 1.2 - Prob. 1.5PCh. 1.2 - Prob. 1.6PCh. 1.3 - Characterize each of the following structures as...Ch. 1.3 - Characterize each of the following structures as...Ch. 1.3 - Characterize each of the following structures as...Ch. 1.3 - Characterize each of the following structures as...Ch. 1.3 - Characterize each of the following structures as...
Knowledge Booster
Similar questions
- Label the spectrum with spectroscopyarrow_forwardQ1: Draw the most stable and the least stable Newman projections about the C2-C3 bond for each of the following isomers (A-C). Are the barriers to rotation identical for enantiomers A and B? How about the diastereomers (A versus C or B versus C)? enantiomers H Br H Br (S) CH3 H3C (S) (R) CH3 H3C H Br A Br H C H Br H3C (R) B (R)CH3 H Br H Br H3C (R) (S) CH3 Br H D identicalarrow_forwardLabel the spectrumarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning