
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
13th Edition
ISBN: 9780134421353
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.46APP
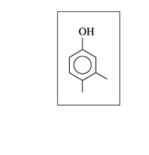
Give the IUPAC name for each of the following alcohols and phenols: (12.1)
a.
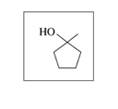
b.
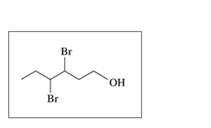
c.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain the term “inductively coupled plasma.”
Using Pauling electronegativity values and a Ketelaar triangle, what type of compound is brass, a CuZn alloy?
Group of answer choices
metallic
ionic
covalent
Challenging samples: 1. Metal complexes with low volatility are often difficult to analyze when performing atomic absorption measurements because the atomization efficiency is reduced to unacceptably low levels. Devise a strategy or strategies for eliminating the problem of a non-volatile metal complex? Explain how you would do that.
2. Devise a strategy to overcome unwanted ionization of the analyte? Explain what it would be.
3. Devise a general method that can be used to account for the presence of unknown matrix effects.
Chapter 12 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Prob. 12.3PPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Give the common name for each of the following: a....Ch. 12.1 - Prob. 12.6PPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Draw the condensed structural formula, or...Ch. 12.2 - Classify each of the following alcohols as primary...Ch. 12.2 - Classify each of the following alcohols as primary...
Ch. 12.2 - Prob. 12.11PPCh. 12.2 - Prob. 12.12PPCh. 12.2 - Give an explanation for each of the following...Ch. 12.2 - Give an explanation for each of the following...Ch. 12.3 - Identify each of the following compounds as an...Ch. 12.3 - Identify each of the following compounds as an...Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the IUPAC name for each of the following: a....Ch. 12.3 - Give the IUPAC name for each of the following: a....Ch. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Prob. 12.27PPCh. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Prob. 12.33PPCh. 12.4 - Prob. 12.34PPCh. 12.4 - Oxybenzone is an effective sunscreen whose...Ch. 12.4 - Avobenzone is a common ingredient in sunscreen....Ch. 12 - Prob. 12.37UTCCh. 12 - The compound frambinone has the taste of...Ch. 12 - A compound called resveratrol is an antioxidant,...Ch. 12 - A compound called cinnamaldehyde is found in...Ch. 12 - Prob. 12.41UTCCh. 12 - Prob. 12.42UTCCh. 12 - Prob. 12.43APPCh. 12 - Classify each of the following alcohols as primary...Ch. 12 - Give the IUPAC name for each of the following...Ch. 12 - Give the IUPAC name for each of the following...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which compound in each pair would be more soluble...Ch. 12 - Which compound in each pair would be more soluble...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Give the IUPAC name for each of the following:...Ch. 12 - Give the IUPAC name for each of the following:...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.63CPCh. 12 - Draw the condensed structural formulas and give...Ch. 12 - A compound with the formula C4H8O is synthesized...Ch. 12 - A compound with the formula C5H10O oxidizes to...Ch. 12 - Compound A is a primary alcohol whose formula is...Ch. 12 - Compound X is a secondary alcohol whose formula is...Ch. 12 - Prob. 21CICh. 12 - Prob. 22CICh. 12 - Prob. 23CICh. 12 - Prob. 24CICh. 12 - Prob. 25CICh. 12 - lonone is a compound that gives violets their...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raitingarrow_forwardDon't used hand raiting don't used Ai solutionarrow_forwardHomework: Atomic Structure This homework is due at the beginning of class next lecture period and is worth 6 points. Please place the number of protons and neutrons in the nucleus and then put the number of electrons in the correct shell. Also give the correct atomic mass. Also, state if the atom is an ion (cation or anion). H* 1. Number of protons Number of electrons Number of neutrons Atomic mass 2. 26 13AI +++ Number of protons Number of neutrons Number of electrons Atomic massarrow_forward
- Don't used Ai solution and don't used hand raitingarrow_forward& Calculate the molar enthalpy of combustion (A combH) of 1.80 g of pyruvic acid (CH3COCOOH; 88.1 g mol-1) at 37 °C when they are combusted in a calorimeter at constant volume with a calorimeter constant = 1.62 kJ °C-1 and the temperature rose by 1.55 °C. Given: R = 8.314 J mol −1 °C-1 and the combustion reaction: AN C3H4O3 + 2.502(g) → 3CO2(g) + 2H2O(l)arrow_forwardAn unknown salt, AB, has the following precipitation reaction:A+(aq) + B-(aq) ⇌ AB(s) the K value for this reaction is 4.50 x10-6. Draw a model that represents what will happen when 1.00 L each of 1.00 M solution of A+(aq) and 1.00M solution of B-(aq) are combined.arrow_forward
- 5. a) Use the rules in Example 4.4 (p. 99) and calculate sizes of octahedral and tetrahedral cavities in titanium and in zirconium. Use values for atomic radii given in Fig. 9.1 (p.291). (3 points) b) Consider the formation of carbides (MC) of these metals. Which metal is able to accommodate carbon atoms better, and which cavities (octahedral or tetrahedral) would be better suited to accommodate C atoms into metal's lattice? (4 points)arrow_forward2. Read paragraph 3.4 in your textbook ("Chiral Molecules"), and explain if Cobalt(ethylenediamine) 33+ shown in previous problem is a chiral species. If yes, draw projections of both enantiomers as mirror images, analogous to mirror projections of hands (below). Mirror (4 points)arrow_forward3. Borane (BH3) belongs to D3h point group. Consider the vibrational (stretching) modes possible for B-H bonds under D3h symmetry. Using the methods we used in class, construct the reducible representation I, and break it down into irreducible representations using the character table provided. Sketch those modes, indicate whether they are IR-active. (6 points) D3h E 2C3 3C2 σh 283 30% A₁' 1 1 1 1 1 1 x² + y², z² 1 -1 1 1 -1 R₂ E' 2 0 2 0 (x, y) (x² - y², xy) " A₁" 1 1 -1 A2" 1 -1 -1 1 Z E" 2 -1 0 -2 1 0 (Ry, Ry) (xz, yz)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY