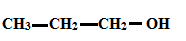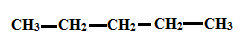Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
13th Edition
ISBN: 9780134421353
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 12.2, Problem 12.12PP
Interpretation Introduction
To determine: Solubility of Alcohols using polarity of the molecule
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a. Write the product For each of the
Following reactions
H
6-836-6
레
+H₂ N
A
H
A-C-C=C-C-CH + 2 Na +2 NH3 -
H H
b. Write the reaction Mechanism For.
reaction
each
help draw the molecule
How to draw this claisen condensation reaction mechanisms/
Chapter 12 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Give the IUPAC name for each of the following: a....Ch. 12.1 - Prob. 12.3PPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Give the common name for each of the following: a....Ch. 12.1 - Prob. 12.6PPCh. 12.1 - Draw the condensed structural formula, or...Ch. 12.1 - Draw the condensed structural formula, or...Ch. 12.2 - Classify each of the following alcohols as primary...Ch. 12.2 - Classify each of the following alcohols as primary...
Ch. 12.2 - Prob. 12.11PPCh. 12.2 - Prob. 12.12PPCh. 12.2 - Give an explanation for each of the following...Ch. 12.2 - Give an explanation for each of the following...Ch. 12.3 - Identify each of the following compounds as an...Ch. 12.3 - Identify each of the following compounds as an...Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the common name for each of the following: a....Ch. 12.3 - Give the IUPAC name for each of the following: a....Ch. 12.3 - Give the IUPAC name for each of the following: a....Ch. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Draw the condensed structural formula for each of...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.3 - Which compound in each of the following pairs...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Write the balanced chemical equation for the...Ch. 12.4 - Prob. 12.27PPCh. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural or line-angle...Ch. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Draw the condensed structural formulas for the...Ch. 12.4 - Prob. 12.33PPCh. 12.4 - Prob. 12.34PPCh. 12.4 - Oxybenzone is an effective sunscreen whose...Ch. 12.4 - Avobenzone is a common ingredient in sunscreen....Ch. 12 - Prob. 12.37UTCCh. 12 - The compound frambinone has the taste of...Ch. 12 - A compound called resveratrol is an antioxidant,...Ch. 12 - A compound called cinnamaldehyde is found in...Ch. 12 - Prob. 12.41UTCCh. 12 - Prob. 12.42UTCCh. 12 - Prob. 12.43APPCh. 12 - Classify each of the following alcohols as primary...Ch. 12 - Give the IUPAC name for each of the following...Ch. 12 - Give the IUPAC name for each of the following...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which compound in each pair would be more soluble...Ch. 12 - Which compound in each pair would be more soluble...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Give the IUPAC name for each of the following:...Ch. 12 - Give the IUPAC name for each of the following:...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Which of the following aldehydes or ketones are...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Draw the condensed structural or line-angle...Ch. 12 - Prob. 12.63CPCh. 12 - Draw the condensed structural formulas and give...Ch. 12 - A compound with the formula C4H8O is synthesized...Ch. 12 - A compound with the formula C5H10O oxidizes to...Ch. 12 - Compound A is a primary alcohol whose formula is...Ch. 12 - Compound X is a secondary alcohol whose formula is...Ch. 12 - Prob. 21CICh. 12 - Prob. 22CICh. 12 - Prob. 23CICh. 12 - Prob. 24CICh. 12 - Prob. 25CICh. 12 - lonone is a compound that gives violets their...
Knowledge Booster
Similar questions
- Write all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forwardHow can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forward
- Please provide the mechanism for this reacitonarrow_forwardQuestion 5: Name the following compound in two ways using side chain and using prefix amine (Common name and IUPAC name both) CH3NH2 CH3CH2NHCH3 CH₂CH₂N(CH3)2 Draw the structure of diethyl methyl amine Question 6. Write the balanced combustion reaction for: a. Hexane b. Propyne c. 2-pentene Question 7: Write the following electrophilic substitution reactions of benzene: Hint: Use notes if you get confused a. Halogenation reaction: b. Nitration reaction : c. Sulphonation reaction: d. Alkylation reaction: e. Aceylation reaction:arrow_forwardQuestion 4. Name the following structures ○ CH3-C-N-H H CH3CH2-C-N-H H CH3CH2-C-N-CH3 Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY