
Concept explainers
Select those compounds that can be correctly called unsaturated and classify each one as an
a.
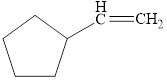
b.
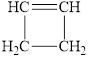
c.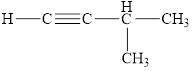 h.
h.
d. i.
i.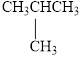
e.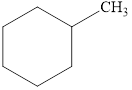
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Bundle: Chemistry for Today: General, Organic, and Biochemistry, Loose-Leaf Version, 9th + LMS Integrated OWLv2, 4 terms (24 months) Printed Access Card
- 1.57 Draw all reasonable resonance structures for the following cation. Then draw the resonance hybrid.arrow_forwardFor the two questions below, draw the mechanism and form the major product.arrow_forwardIndicate similarities and differences between natural, exchanged and pillared clays.arrow_forward
- Show work. don't give Ai generated solutionarrow_forwardIn intercalation compounds, their sheets can be neutral or have a negative or positive charge, depending on the nature of the incorporated species and its structure. Is this statement correct?arrow_forwardThis thermodynamic cycle describes the formation of an ionic compound MX2 from a metal element M and nonmetal element X in their standard states. What is the lattice enthalpy of MX2 ? What is the enthalpy formation of MX2 ? Suppose both the heat of sublimation of M and the ionization enthalpy of M were smaller. Would MX2 be more stable? Or less? or impossible to tell without more information?arrow_forward
- I need to make 25mL of solution with the stocks shown below. How would I calculate the math?arrow_forwardWe are practicing calculating for making solutions. How would I calculate this?arrow_forwardBr. , H+ .OH Mg ether solvent H+, H₂O 17. Which one of the compounds below is the final product of the reaction sequence shown above? HO A HO HO OH D B OH HO OH C OH HO OH Earrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





