
Connect 2-Year Online Access for General, Organic, and Biochemistry
9th Edition
ISBN: 9781259677946
Author: Denniston
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Question
Chapter 12, Problem 12.33QP
(a)
Interpretation Introduction
Interpretation:
IUPAC name for the given compound has to be given.
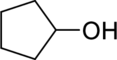
Concept Introduction:
A common nomenclature of naming organic compounds has been developed by IUPAC. By usage of this nomenclature or rules, memorizing of names of organic compounds is not necessary.
IUPAC rules for naming alcohols:
- Alcohols are named by identifying the parent compound and replacing the –e ending with –ol.
- Lowest possible number should be given for the hydroxyl group present in the parent chain.
- Name and number all substituent, and put them as prefixes to “alkanol” name also give the first preference as per the alphabetical order.
- If an alcohol contains two hydroxyl groups then name it as
diol , an alcohol; contains three hydroxyl groups then name it as triol.
(b)
Interpretation Introduction
Interpretation:
IUPAC name for the given compound has to be given.
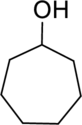
Concept Introduction:
Refer part (a).
(c)
Interpretation Introduction
Interpretation:
IUPAC name for the given compound has to be given.
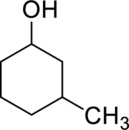
Concept Introduction:
Refer part (a).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a tetramer if this alternating copolymer please
Draw the monomers required to synthesize this condensation polymer.
Draw the monomers required to synthesize this condensation polymer.
Chapter 12 Solutions
Connect 2-Year Online Access for General, Organic, and Biochemistry
Ch. 12.1 - Prob. 12.1PPCh. 12.2 - Prob. 12.2PPCh. 12.2 - Prob. 12.3PPCh. 12.2 - Prob. 12.1QCh. 12.2 - Prob. 12.2QCh. 12.4 - Write an equation representing the reduction of...Ch. 12.4 - Write an equation representing the reduction of...Ch. 12.4 - Prob. 12.6PPCh. 12.4 - Prob. 12.7PPCh. 12.4 - Prob. 12.8PP
Ch. 12.4 - Prob. 12.3QCh. 12.4 - Prob. 12.4QCh. 12.4 - Prob. 12.5QCh. 12.4 - Prob. 12.6QCh. 12.4 - Prob. 12.7QCh. 12.4 - Prob. 12.8QCh. 12.6 - Prob. 12.9QCh. 12.6 - Prob. 12.10QCh. 12.7 - Name the following ethers using IUPAC...Ch. 12.7 - Prob. 12.10PPCh. 12.7 - Prob. 12.11PPCh. 12.7 - Prob. 12.11QCh. 12.7 - Prob. 12.12QCh. 12.8 - Prob. 12.12PPCh. 12 - Prob. 12.13QPCh. 12 - Prob. 12.14QPCh. 12 - Prob. 12.15QPCh. 12 - Prob. 12.16QPCh. 12 - Prob. 12.17QPCh. 12 - Prob. 12.18QPCh. 12 - Prob. 12.19QPCh. 12 - Prob. 12.20QPCh. 12 - Stingless bees use complex systems to communicate....Ch. 12 - Prob. 12.22QPCh. 12 - Prob. 12.23QPCh. 12 - Prob. 12.24QPCh. 12 - Prob. 12.25QPCh. 12 - Prob. 12.26QPCh. 12 - Prob. 12.27QPCh. 12 - Prob. 12.28QPCh. 12 - Prob. 12.29QPCh. 12 - Prob. 12.30QPCh. 12 - Prob. 12.31QPCh. 12 - Prob. 12.32QPCh. 12 - Prob. 12.33QPCh. 12 - Prob. 12.34QPCh. 12 - Prob. 12.35QPCh. 12 - Prob. 12.36QPCh. 12 - Prob. 12.37QPCh. 12 - Prob. 12.38QPCh. 12 - Prob. 12.39QPCh. 12 - Prob. 12.40QPCh. 12 - Prob. 12.41QPCh. 12 - Prob. 12.42QPCh. 12 - Prob. 12.43QPCh. 12 - Prob. 12.44QPCh. 12 - Prob. 12.45QPCh. 12 - Prob. 12.46QPCh. 12 - Prob. 12.47QPCh. 12 - Prob. 12.48QPCh. 12 - Prob. 12.49QPCh. 12 - Prob. 12.50QPCh. 12 - Prob. 12.51QPCh. 12 - Prob. 12.52QPCh. 12 - Prob. 12.53QPCh. 12 - Prob. 12.54QPCh. 12 - Prob. 12.55QPCh. 12 - Prob. 12.56QPCh. 12 - Prob. 12.57QPCh. 12 - Prob. 12.58QPCh. 12 - Prob. 12.59QPCh. 12 - Prob. 12.60QPCh. 12 - Prob. 12.61QPCh. 12 - Prob. 12.62QPCh. 12 - Prob. 12.63QPCh. 12 - Prob. 12.64QPCh. 12 - Prob. 12.65QPCh. 12 - Prob. 12.66QPCh. 12 - Prob. 12.67QPCh. 12 - Prob. 12.68QPCh. 12 - Prob. 12.69QPCh. 12 - Prob. 12.70QPCh. 12 - Prob. 12.71QPCh. 12 - Prob. 12.72QPCh. 12 - Prob. 12.73QPCh. 12 - Prob. 12.74QPCh. 12 - Prob. 12.75QPCh. 12 - Prob. 12.76QPCh. 12 - Prob. 12.77QPCh. 12 - Prob. 12.78QPCh. 12 - Prob. 12.79QPCh. 12 - Prob. 12.80QPCh. 12 - Prob. 12.81QPCh. 12 - Prob. 12.82QPCh. 12 - Prob. 12.83QPCh. 12 - Prob. 12.84QPCh. 12 - Prob. 12.85QPCh. 12 - Write the IUPAC and common name for each of the...Ch. 12 - Prob. 12.87QPCh. 12 - Prob. 12.88QPCh. 12 - Prob. 12.89QPCh. 12 - Prob. 12.90QPCh. 12 - Prob. 12.91QPCh. 12 - Prob. 12.92QPCh. 12 - Prob. 1CPCh. 12 - Prob. 4CPCh. 12 - Prob. 5CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forwardi need help with the folarrow_forward
- no AI walkthrough current image is wrong answerarrow_forwarda. Determine whether each of the Followery Molecules is in the R- On the y- Configuration 1-01"/ 1-6-4 Br 4 I el Br b. Draw The Fisher projection For all the Meso compounds that can exist FOR The Following molenlearrow_forward1- Refer to the monosaccharides below to answer each of the following question(s): CH₂OH CHO CH₂OH CH₂OH 0 H- OH 0 0 HO- H H- -OH HO H HO H H OH HO- H CH₂OH H. OH HO H HO- H CH₂OH CH₂OH CH3 a. Sorbose b. Rhamnose c. Erythrulose d. Xylulose Classify each sugar by type; for example, glucose is an aldohexose. a. Xylulose is .. b. Erythrulose is . c. Sorbose is .. d. Rhamnose is .. 2- Consider the reaction below to answer the following question(s). CHO H OH CH₂OH CH₂OH HO- H HO HO + H. -OH HO OH HO. H OH OH H -OH H OH CH₂OH Q Z a. Refer to Exhibit 25-11. Place a triangle around the anomeric carbon in compound Q. Compound Z is: b. 1. the D-anomer. 2. the a-anomer. 3. the ẞ-anomer. 4. the L-anomer. c. Which anomer is the LEAST stable? d. Q and Z are cyclic examples of: a. acetals b. hemiacetals c. alditols d. hemialditolsarrow_forward
- i need help identifying the four carbon oxygen bonds in the following:arrow_forwardImagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule HO H3N + The solution is... X O acidic OH O basic H3N-CH-C-O O neutral ○ (unknown) O acidic ○ basic CH2 CH 3-S-CH2 O neutral ○ (unknown) H3N O OH O acidic O basic Oneutral O (unknown) 0 H3N-CH-C-O CH3 CH CH3 O acidic O basic O neutral ○ (unknown) ? olo Ar BHarrow_forwardno Ai walkthroughs need other product (product in picture is wrong dont submit the same thing)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY