![OWLv2 for Moore/Stanitski's Chemistry: The Molecular Science, 5th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
OWLv2 for Moore/Stanitski's Chemistry: The Molecular Science, 5th Edition, [Instant Access], 1 term (6 months)
5th Edition
ISBN: 9781285460420
Author: John W. Moore; Conrad L. Stanitski
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 112QRT
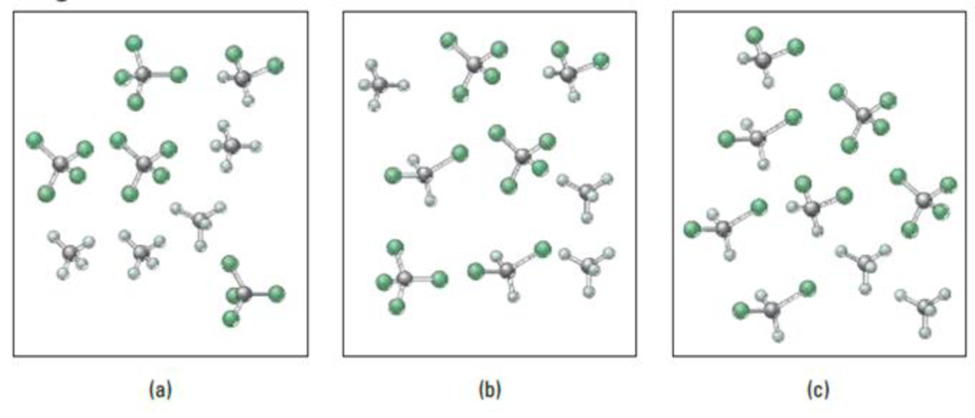
For the reaction in Question 111, which diagram for Question 111 represents an equilibrium mixture at
- (a) a temperature below 350 K?
- (b) a temperature above 350 K?
Diagrams for Question 111.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
please solve this problem by telling me which boxes to check. Thank you in advance!
Explain what characteristics of metalloids are more like metals and which are more like nonmetals, based on Na, Mg, Fe, Cl, and Ar.
please solve this, and help me know which boxes to check. Thank you so much in advance.
Chapter 12 Solutions
OWLv2 for Moore/Stanitski's Chemistry: The Molecular Science, 5th Edition, [Instant Access], 1 term (6 months)
Ch. 12.1 - The introduction to this chapter states that at a...Ch. 12.1 - Prob. 12.2CECh. 12.2 - After a mixture of cis-2-butene and trans-2-butene...Ch. 12.2 - Prob. 12.1PSPCh. 12.2 - Prob. 12.4ECh. 12.2 - When carbon dioxide dissolves in water it reacts...Ch. 12.2 - For each of these reactions, calculate KP from Kc....Ch. 12.3 - Prob. 12.3PSPCh. 12.4 - Suppose that solid AgCl and AgI are placed in 1.0...Ch. 12.4 - Prob. 12.6CE
Ch. 12.5 - For the equilibrium 2 SO2(g) + O2(g) 2 SO3(g) Kc...Ch. 12.5 - Prob. 12.7CECh. 12.5 - Prob. 12.6PSPCh. 12.5 - Prob. 12.7PSPCh. 12.6 - Prob. 12.8CECh. 12.6 - Prob. 12.9ECh. 12.6 - Prob. 12.10CECh. 12.6 - Prob. 12.8PSPCh. 12.7 - For the ammonia synthesis reaction
⇌
Does the...Ch. 12.8 - Prob. 12.13CECh. 12 - Prob. 1QRTCh. 12 - Prob. 2QRTCh. 12 - Prob. 3QRTCh. 12 - Decomposition of ammonium dichromate is shown in...Ch. 12 - For the equilibrium reaction in Question 4, write...Ch. 12 - Indicate whether each statement below is true or...Ch. 12 - Prob. 7QRTCh. 12 - Prob. 8QRTCh. 12 - Prob. 9QRTCh. 12 - Prob. 10QRTCh. 12 - The atmosphere consists of about 80% N2 and 20%...Ch. 12 - Prob. 12QRTCh. 12 - Prob. 13QRTCh. 12 - Prob. 14QRTCh. 12 - Prob. 15QRTCh. 12 - Prob. 16QRTCh. 12 - Prob. 17QRTCh. 12 - Prob. 18QRTCh. 12 - Prob. 19QRTCh. 12 - Prob. 20QRTCh. 12 - Prob. 21QRTCh. 12 - Prob. 22QRTCh. 12 - Prob. 23QRTCh. 12 - Prob. 24QRTCh. 12 - Prob. 25QRTCh. 12 - Prob. 26QRTCh. 12 - Prob. 27QRTCh. 12 - Prob. 28QRTCh. 12 - Prob. 29QRTCh. 12 - Prob. 30QRTCh. 12 - Given these data at a certain temperature,...Ch. 12 - The vapor pressure of water at 80. C is 0.467 atm....Ch. 12 - Prob. 33QRTCh. 12 - Prob. 34QRTCh. 12 - Prob. 35QRTCh. 12 - Prob. 36QRTCh. 12 - Carbon dioxide reacts with carbon to give carbon...Ch. 12 - Prob. 38QRTCh. 12 - Prob. 39QRTCh. 12 - Prob. 40QRTCh. 12 - Nitrosyl chloride, NOC1, decomposes to NO and Cl2...Ch. 12 - Suppose 0.086 mol Br2 is placed in a 1.26-L flask....Ch. 12 - Prob. 43QRTCh. 12 - Prob. 44QRTCh. 12 - Prob. 45QRTCh. 12 - Using the data of Table 12.1, predict which of...Ch. 12 - Prob. 47QRTCh. 12 - The equilibrium constants for dissolving silver...Ch. 12 - Prob. 49QRTCh. 12 - Prob. 50QRTCh. 12 - At room temperature, the equilibrium constant Kc...Ch. 12 - Prob. 52QRTCh. 12 - Consider the equilibrium N2(g)+O2(g)2NO(g) At 2300...Ch. 12 - The equilibrium constant, Kc, for the reaction...Ch. 12 - Prob. 55QRTCh. 12 - Prob. 56QRTCh. 12 - Prob. 57QRTCh. 12 - At 503 K the equilibrium constant Kc for the...Ch. 12 - Prob. 59QRTCh. 12 - Prob. 60QRTCh. 12 - Prob. 61QRTCh. 12 - Prob. 62QRTCh. 12 - Prob. 63QRTCh. 12 - Prob. 64QRTCh. 12 - Prob. 65QRTCh. 12 - Prob. 66QRTCh. 12 - Prob. 67QRTCh. 12 - Hydrogen, bromine, and HBr in the gas phase are in...Ch. 12 - Prob. 69QRTCh. 12 - Prob. 70QRTCh. 12 - Prob. 71QRTCh. 12 - Prob. 72QRTCh. 12 - Prob. 73QRTCh. 12 - Prob. 74QRTCh. 12 - Consider the system
4 NH3(g) + 3 O2(g) ⇌ 2 N2(g) +...Ch. 12 - Prob. 76QRTCh. 12 - Predict whether the equilibrium for the...Ch. 12 - Prob. 78QRTCh. 12 - Prob. 79QRTCh. 12 - Prob. 80QRTCh. 12 - Prob. 81QRTCh. 12 - Prob. 82QRTCh. 12 - Prob. 83QRTCh. 12 - Prob. 84QRTCh. 12 - Prob. 85QRTCh. 12 - Prob. 86QRTCh. 12 - Prob. 87QRTCh. 12 - Consider the decomposition of ammonium hydrogen...Ch. 12 - Prob. 89QRTCh. 12 - Prob. 90QRTCh. 12 - Prob. 91QRTCh. 12 - Prob. 92QRTCh. 12 - Prob. 93QRTCh. 12 - Prob. 94QRTCh. 12 - Prob. 95QRTCh. 12 - Prob. 96QRTCh. 12 - Prob. 97QRTCh. 12 - Prob. 98QRTCh. 12 - Prob. 99QRTCh. 12 - Prob. 100QRTCh. 12 - Two molecules of A react to form one molecule of...Ch. 12 - Prob. 102QRTCh. 12 - In Table 12.1 (←Sec. 12-3a) the equilibrium...Ch. 12 - Prob. 104QRTCh. 12 - Prob. 105QRTCh. 12 - Prob. 106QRTCh. 12 - Prob. 107QRTCh. 12 - Which of the diagrams for Questions 107 and 108...Ch. 12 - Draw a nanoscale (particulate) level diagram for...Ch. 12 -
The diagram represents an equilibrium mixture for...Ch. 12 - The equilibrium constant, Kc, is 1.05 at 350 K for...Ch. 12 - For the reaction in Question 111, which diagram...Ch. 12 - Prob. 113QRTCh. 12 - Prob. 114QRTCh. 12 - Prob. 115QRTCh. 12 - For the equilibrium...Ch. 12 - Prob. 117QRTCh. 12 - Prob. 119QRTCh. 12 - Prob. 120QRTCh. 12 - When a mixture of hydrogen and bromine is...Ch. 12 - Prob. 122QRTCh. 12 - Prob. 123QRTCh. 12 - Prob. 124QRTCh. 12 - Prob. 125QRTCh. 12 - Prob. 12.ACPCh. 12 - Prob. 12.BCPCh. 12 - Prob. 12.CCPCh. 12 - Prob. 12.DCPCh. 12 - Prob. 12.ECPCh. 12 - Prob. 12.FCP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Describe how electronegativity is illustrated on the periodic table including trends between groups and periods and significance of atom size.arrow_forwardDefine the term “transition.” How does this definition apply to the transition metals?arrow_forwardDescribe how the properties of the different types of elements (metals, nonmetals, metalloids) differ.arrow_forward
- Use a textbook or other valid source to research the physical and chemical properties of each element listed in Data Table 1 using the following as a guideline: Ductile (able to be deformed without losing toughness) and malleable (able to be hammered or pressed permanently out of shape without breaking or cracking) or not ductile or malleable Good, semi, or poor conductors of electricity and heat High or low melting and boiling points Occur or do not occur uncombined/freely in nature High, intermediate, or low reactivity Loses or gains electrons during reactions or is not reactivearrow_forwardProvide the Physical and Chemical Properties of Elements of the following elements listedarrow_forwardQuestions 4 and 5arrow_forward
- For a titration of 40.00 mL of 0.0500 M oxalic acid H2C2O4 with 0.1000 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin;2) 15 mL; 3) 20 mL; 4) 25 mL; 5) 40 mL; 6) 50 mL. Ka1 = 5.90×10^-2, Ka2 = 6.50×10^-5 for oxalic acid.arrow_forwardPredict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.arrow_forwardPredict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.arrow_forward
- How many signals would you expect to find in the 1 H NMR spectrum of each given compound? Part 1 of 2 2 Part 2 of 2 HO 5 ☑ Х IIIIII***** §arrow_forwardA carbonyl compound has a molecular ion with a m/z of 86. The mass spectra of this compound also has a base peak with a m/z of 57. Draw the correct structure of this molecule. Drawingarrow_forwardCan you draw this using Lewis dot structures and full structures in the same way they are so that I can better visualize them and then determine resonance?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY