
Interpretation:
The formation of
Concept introduction:
Elimination reaction: An elimination reaction is removal of two substituents in a molecule and forms alkene. An elimination reaction is one or two-step process which based on the mechanism when two substituents removed from the molecule in single step is called E2 reaction. When two substituents are removed from the molecule in two steps is called E1 reaction.
E2 elimination:Alkyl halide forms an alkene from the abstraction of the proton from the β-carbon atom followed by elimination of the bromine in a single step.
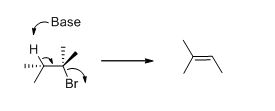
Given information:
The given compound is shown below,
Trending nowThis is a popular solution!

Chapter 11 Solutions
Organic Chemistry - With Access (Custom)
- (c) (4pts) Mechanism: heat (E1) CH3OH + 1.5pts each _E1 _ (1pt) Br CH3OH (d) (4pts) Mechanism: SN1 (1pt) (e) (3pts) 1111 I H 10 Ill!! H LDA THF (solvent) Mechanism: E2 (1pt) NC (f) Bri!!!!! CH3 NaCN (3pts) acetone Mechanism: SN2 (1pt) (SN1) -OCH3 OCH3 1.5pts each 2pts for either product 1pt if incorrect stereochemistry H Br (g) “,、 (3pts) H CH3OH +21 Mechanism: SN2 (1pt) H CH3 2pts 1pt if incorrect stereochemistry H 2pts 1pt if incorrect stereochemistryarrow_forwardA mixture of butyl acrylate and 4'-chloropropiophenone has been taken for proton NMR analysis. Based on this proton NMR, determine the relative percentage of each compound in the mixturearrow_forwardQ5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

