
Concept explainers
(a)
Interpretation:
The resultant product obtained from the metathesis of the alkene
Concept introduction:
Alkene Metathesis Or olefin metathesis
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
(b)
Interpretation:
The resultant product obtained from the metathesis of the following alkene should be identified.
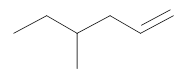
Concept introduction:
Alkene Metathesis Or olefin metathesis
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
(c)
Interpretation:
The resultant product obtained from the metathesis of the following alkene should be identified.
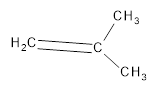
Concept introduction:
Alkene Metathesis Or (olefin metathesis)
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
(d)
Interpretation:
The resultant product obtained from the metathesis of the following alkene should be identified.
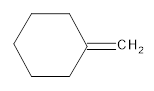
Concept introduction:
Alkene Metathesis Or (olefin metathesis)
This breaks the double bond of an alkene and then rejoins the fragments. When the fragments are joined, the new double bond is formed between two
Terminal alkene gives the best yields of a single alkene product in metathesis because one of the products is ethane, which is equally removed from the reaction mixture, thus shifting the equilibrium in favor of the other new alkene product.
Trending nowThis is a popular solution!

Chapter 11 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
- What is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forward
- What is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forward
- What would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



