
Organic Chemistry, Loose-leaf Version
8th Edition
ISBN: 9781305865549
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.5, Problem 11.6P
Draw structural formulas for the major products of each reaction.
(a) 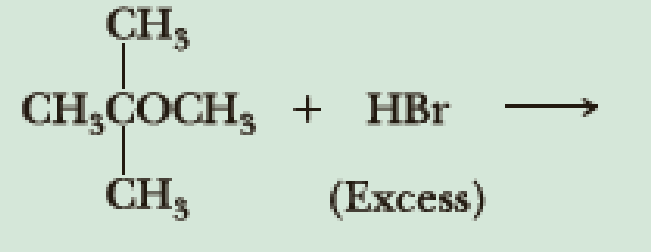
(b) 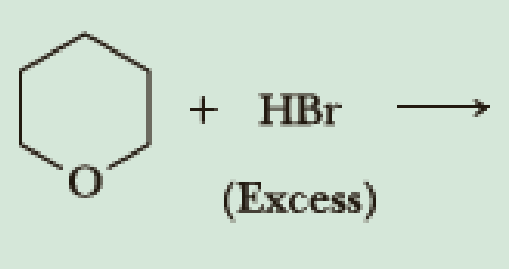
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Predict the intermediate 1 and final product 2 of this organic reaction:
NaOMe
ག1, ད།་, -
+
H
You can draw 1 and 2 in any arrangement you like.
2
work up
Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge)
bonds at the chiral center.
Explanation
Check
Click and drag to start drawing a structure.
Х
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | P
What is the total energy cost associated with the compound below adopting the shown conformation?
CH3
HH
DH
CH3
ΗΝ,
Draw Final Product
C
cyclohexanone
pH 4-5
Edit Enamine
H3O+
CH3CH2Br
THF, reflux
H
Edit Iminium Ion
Chapter 11 Solutions
Organic Chemistry, Loose-leaf Version
Ch. 11.2 - Write IUPAC and common names for these ethers. (a)...Ch. 11.3 - Arrange these compounds in order of increasing...Ch. 11.4 - Show how you might use the Williamson ether...Ch. 11.4 - Show how ethyl hexyl ether might be prepared by a...Ch. 11.5 - Account for the fact that treatment of tert-butyl...Ch. 11.5 - Draw structural formulas for the major products of...Ch. 11.6 - Prob. 11.7PCh. 11.8 - Draw the expected products of Sharpless...Ch. 11.9 - Prob. AQCh. 11.9 - Prob. BQ
Ch. 11.9 - Prob. CQCh. 11.9 - Prob. DQCh. 11 - Write names for these compounds. Where possible,...Ch. 11 - Prob. 11.11PCh. 11 - Each compound given in this problem is a common...Ch. 11 - Account for the fact that tetrahydrofuran (THF) is...Ch. 11 - Prob. 11.14PCh. 11 - Write equations to show a combination of reactants...Ch. 11 - Propose a mechanism for this reaction.Ch. 11 - Prob. 11.17PCh. 11 - Prob. 11.18PCh. 11 - Prob. 11.19PCh. 11 - Prob. 11.20PCh. 11 - Ethylene oxide is the starting material for the...Ch. 11 - Prob. 11.22PCh. 11 - Predict the structural formula of the major...Ch. 11 - The following equation shows the reaction of...Ch. 11 - Propose a mechanism to account for this...Ch. 11 - Acid-catalyzed hydrolysis of the following epoxide...Ch. 11 - Prob. 11.27PCh. 11 - Prob. 11.28PCh. 11 - Prob. 11.29PCh. 11 - Propose a mechanism for the following...Ch. 11 - Show reagents and experimental conditions to...Ch. 11 - Starting with cis-3-hexene, show how to prepare...Ch. 11 - Show reagents to convert cycloheptene to each of...Ch. 11 - Show reagents to convert bromocyclopentane to each...Ch. 11 - Prob. 11.35PCh. 11 - Starting with acetylene and ethylene oxide as the...Ch. 11 - Following are the steps in the industrial...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - Aldehydes and ketones react with one molecule of...Ch. 11 - Prob. 11.42PCh. 11 - Write the products of the following sequences of...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - During the synthesis of the antiasthmatic drug...Ch. 11 - Prob. 11.48P
Additional Science Textbook Solutions
Find more solutions based on key concepts
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Give the IUPAC name for each compound.
Organic Chemistry
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forward
- H H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forward
- choose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forward
- OH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY