
Show reagents and experimental conditions to synthesize the following compounds from 1-propanol (any derivative of 1-propanol prepared in one part of this problem may be used for the synthesis of another part of the problem).
- (a) Propanal
- (b) Propanoic acid
- (c) Propene
- (d) 2-Propanol
- (e) 2-Bromopropane
- (f) 1-Chloropropane
- (g) 1,2-Dibromopropane
- (h) Propyne
- (i) 2-Propanone
- (j) 1-Chloro-2-propanol
- (k) Methyloxirane
- (l) Dipropyl ether
- (m) Isopropyl propyl ether
- (n) 1-Mercapto-2-propanol
- (o) 1-Amino-2-propanol
- (p) 1,2-Propanediol
(a)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Oxidation of primary alcohol to aldehyde: A Primary alcohol is oxidized to an aldehyde by PCC.

Explanation of Solution
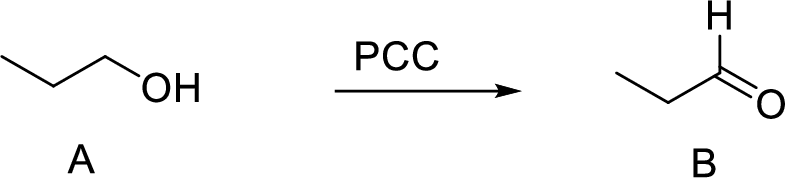
The oxidation of primary alcohol to aldehyde is performed using PCC gives rise to the desired aldehyde (Propanal) product as shown above.
(b)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Hydroboration-oxidation: Generally alkenes react with boron followed by hydrogen peroxide and in presence of base like sodium hydroxide it gives corresponding alcohol and it follows anti-Markovnikov rule.
Oxidation of primary alcohol to aldehyde: A Primary alcohol is oxidized to an aldehyde by PCC.

Explanation of Solution
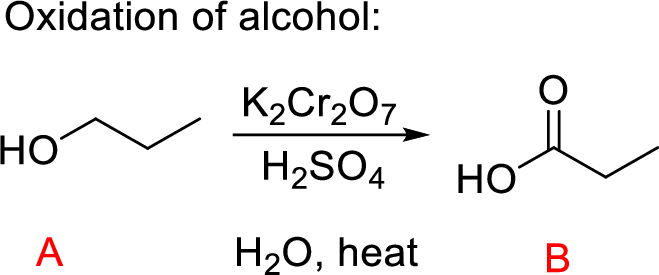
The oxidation of primary alcohol (A) to carboxylic acid (C) is performed using chromic acid on heating; the aldehyde with water in the solution g9ives hydrated aldehyde intermediate leads to the desired Propanoic acid product as shown above.
(c)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of alcohols: An alcohol can be converted into an alkene by dehydration (with elimination of water from adjacent carbon). By heating alcohol with either 85% phosphoric acid or concentrated sulfuric acid. Primary alcohol require high temperature as high as
Ease of dehydration of alcohols:
The ease of acid-catalyzed dehydration of alcohols is in the order as follows,
Explanation of Solution
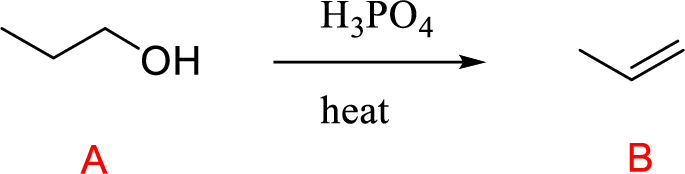
The alcohol (A) compound undergoes acid-catalyzed dehydration
Acid-catalyzed dehydration reaction of alcohol with
(d)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of alcohols: An alcohol can be converted into an alkene by dehydration (with elimination of water from adjacent carbon). By heating alcohol with either 85% phosphoric acid or concentrated sulfuric acid. Primary alcohol require high temperature as high as
Ease of dehydration of alcohols:
The ease of acid-catalyzed dehydration of alcohols is in the order as follows,
Acid Catalyzed Hydration Reaction: The reaction involves breaking of pi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Explanation of Solution
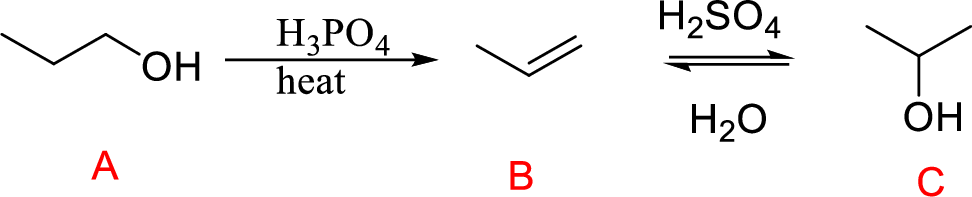
In the above reaction, A to B involves acid-catalyzed dehydration process; followed by acid-catalyzed hydration process (B to C).
Acid-catalyzed hydration:
Initially
Finally, the oxonium ion removes one proton gives the secondary alcohol as the major product.
(e)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Oxidation of secondary alcohol to aldehyde: A secondary alcohol is oxidized to a aldehyde by chromic acid. The mechanism involves initial formation of an alkyl chromate intermediate, followed by reaction with base to remove a proton, generating the carbonyl group of an aldehyde reducing the chromium (VI) to chromium (IV).
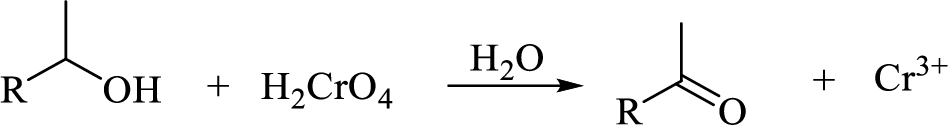
Explanation of Solution
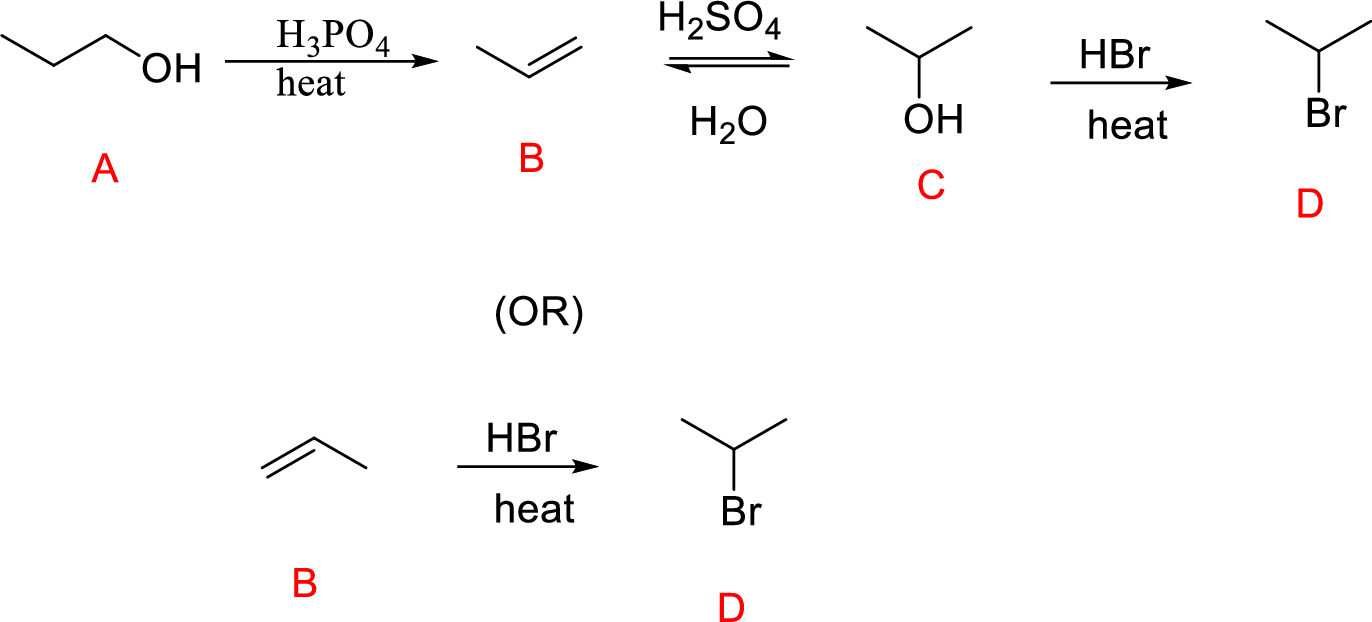
The Propanol undergoes acid-catalyzed dehydration; further acid-catalyzed hydration forming secondary alcohol (C), reaction of secondary alcohol with
(or)
Reaction of Compound (B) reacts with
(f)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Conversion of alcohol into haloalkane:
Conversion of primary and secondary alcohols to Chloroalkane is thionyl chloride. The reaction is carried out in the presence of base such as pyridine or trimethylamine.
The reaction of an alcohol with thionyl chloride is the formation of an alkyl Chlorosulfite that converts hydroxide ion (poor leaving group) into Chlorosulfite (good leaving group). Nucleophilic displacement of this leaving group gives product.
Leaving group: Leaving group can be any groups or atoms that get detached from either neutral or charged organic compounds. The stability of the leaving group is to stabilize the electron density that results from heterolysis cleavage of bond.
Explanation of Solution
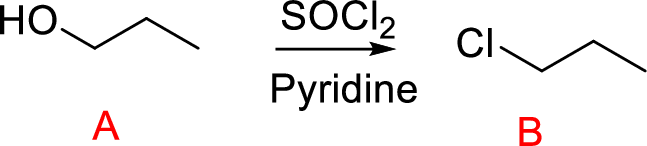
Reaction of alcohol (A) with
(g)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Addition of halogen across double bond: The reaction of alkene with halogen molecules undergoes electrophilic addition reaction forming 1,2-dihaloalkane compound.
Explanation of Solution
The reaction is shown below,
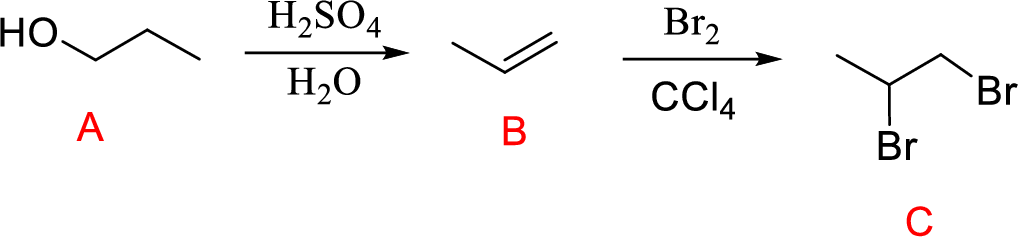
Acid-catalyzed dehydration reaction of alcohol with
Further reaction of alkene by addition of bromine across double bond gives 1,2-dibromopropane.
(h)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Addition of halogen across double bond: The reaction of alkene with halogen molecules undergoes electrophilic addition reaction forming 1,2-dihaloalkane compound.
Explanation of Solution
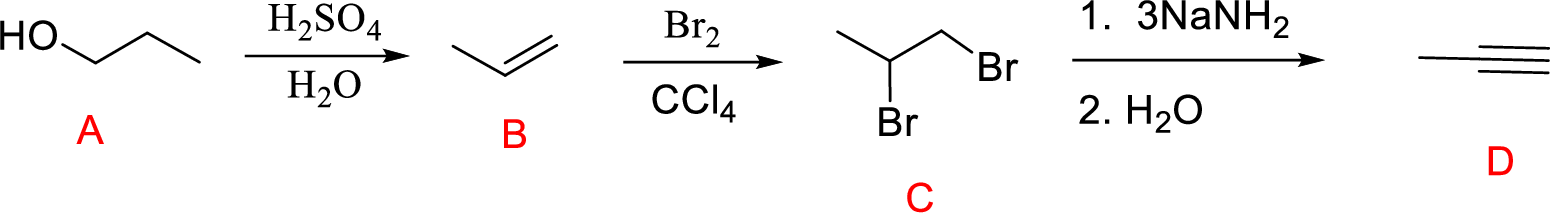
Acid-catalyzed dehydration reaction of alcohol with
Further reaction of alkene by addition of bromine across double bond gives 1,2-dibromopropane.
Addition of strong base leads to the elimination of two moles of
(i)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Explanation of Solution
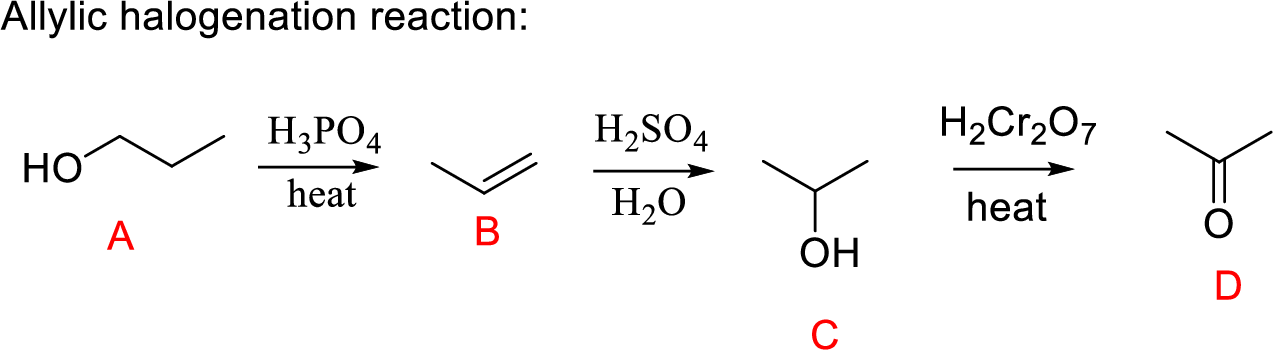
Propanol (A) undergoes dehydration followed by hydration gives secondary alcohol; the oxidation of secondary alcohol to ketone (D) the desired product.
(j)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Addition of halogen across double bond: The reaction of alkene with halogen molecules undergoes electrophilic addition reaction forming 1,2-dihaloalkane compound.
Explanation of Solution
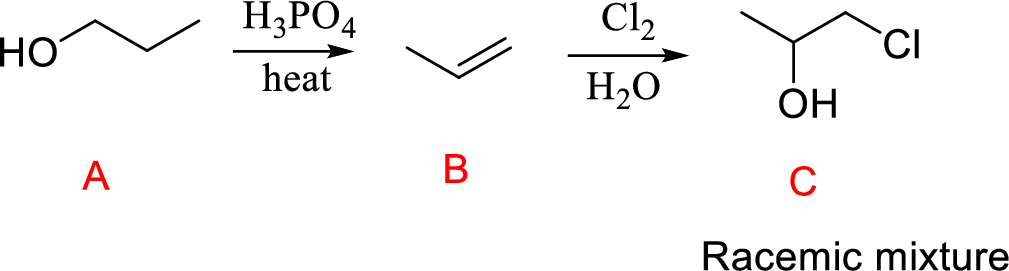
Propanol (A) reacts with phosphoric acid undergoes acid-catalyzed dehydration forming compound (B); further reaction with chlorine molecule leads to the compound (B); further reaction of compound (C).
(k)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept-Introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Formation of epoxide: The alkene can be converted to epoxide when alkene is treated with MCPBA (m-Chloro per benzoic acid)

Explanation of Solution
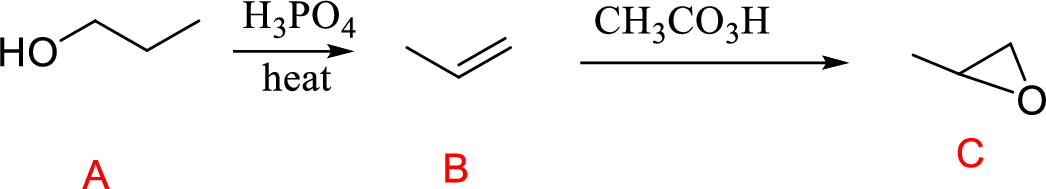
Propanol (A) with phosphoric acid on heating gives alkene compound (B) and further reaction with peroxyacid leads to the formation of epoxide compound (C).
(l)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept-Introduction:
Williamson ether synthesis: A general method for the synthesis of dialkyl ethers by a
Yield of ether becomes highest when the halide to be displaced is on primary carbon; yields are low in the displacement from secondary halides (because of
Explanation of Solution
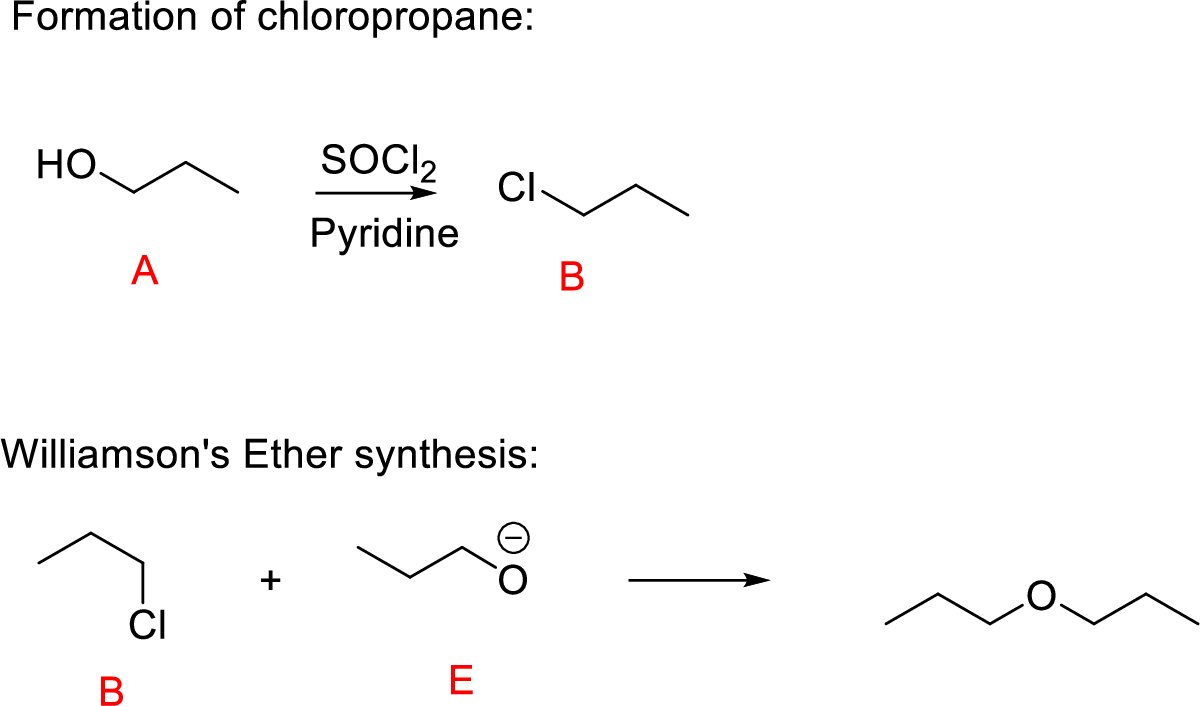
Propanol (A) reacts with
(m)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept-Introduction:
Williamson ether synthesis: A general method for the synthesis of dialkyl ethers by a
Yield of ether becomes highest when the halide to be displaced is on primary carbon; yields are low in the displacement from secondary halides (because of
Explanation of Solution
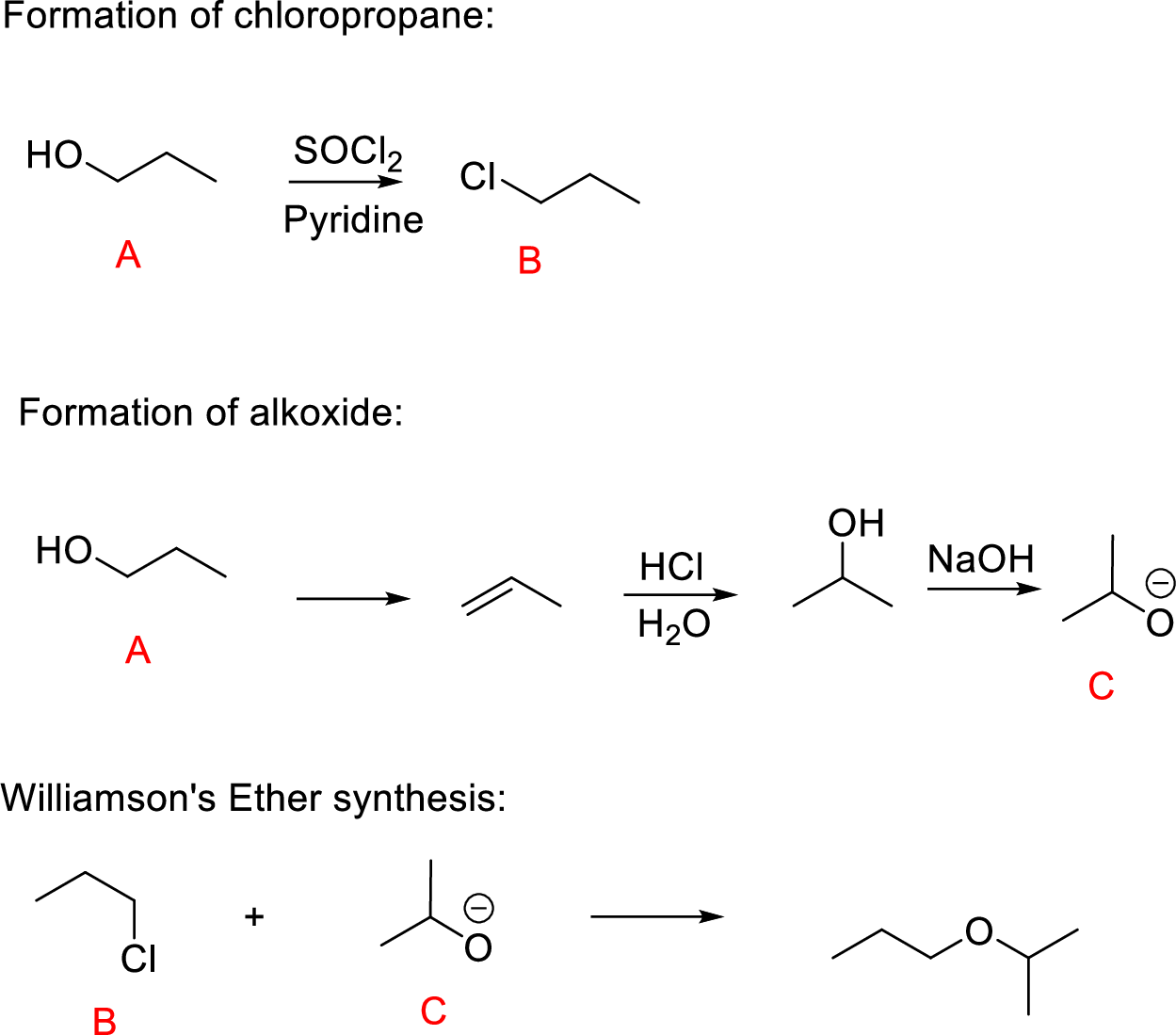
Propanol (A) reacts with
The reaction of compound (B) and compound (C) undergoes Williamson’s ether synthesis forming ether the desired product.
(n)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept-Introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Formation of epoxide: The alkene can be converted to epoxide when alkene is treated with MCPBA (m-Chloro per benzoic acid)

Explanation of Solution
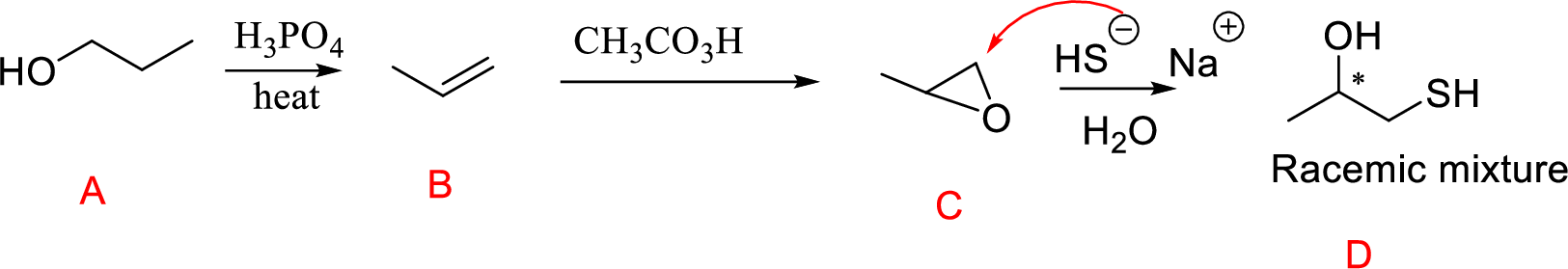
Propanol (A) with phosphoric acid on heating gives alkene compound (B) and further reaction with peroxyacid leads to the formation of epoxide compound (C).
The epoxide ring opening using the nucleophile
(o)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept Introduction:
Acid-catalyzed dehydration of primary and secondary alcohols: Dehydration of primary and secondary alcohols is often accompanied by rearrangement process (shift of hydride or alkyl from
Formation of epoxide: The alkene can be converted to epoxide when alkene is treated with MCPBA (m-Chloro per benzoic acid)

Explanation of Solution
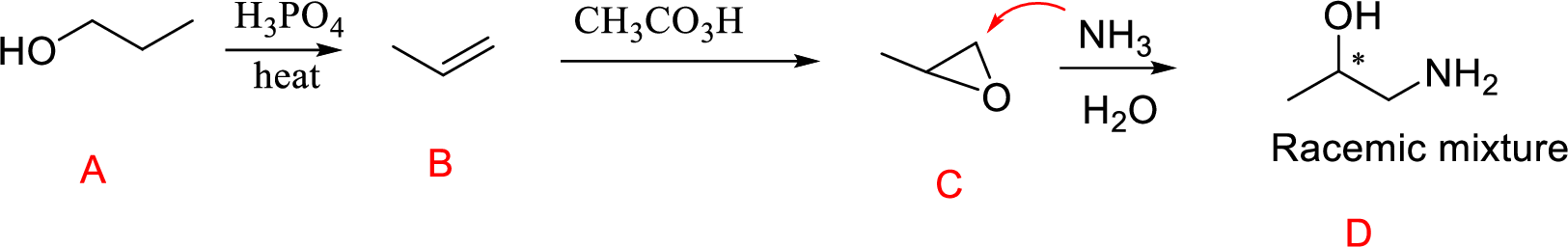
Propanol (A) with phosphoric acid on heating gives alkene compound (B) and further reaction with peroxyacid leads to the formation of epoxide compound (C).
The epoxide ring opening using the nucleophile
(p)
Interpretation:
Conversion of Propanol to given desired product has to be shown.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
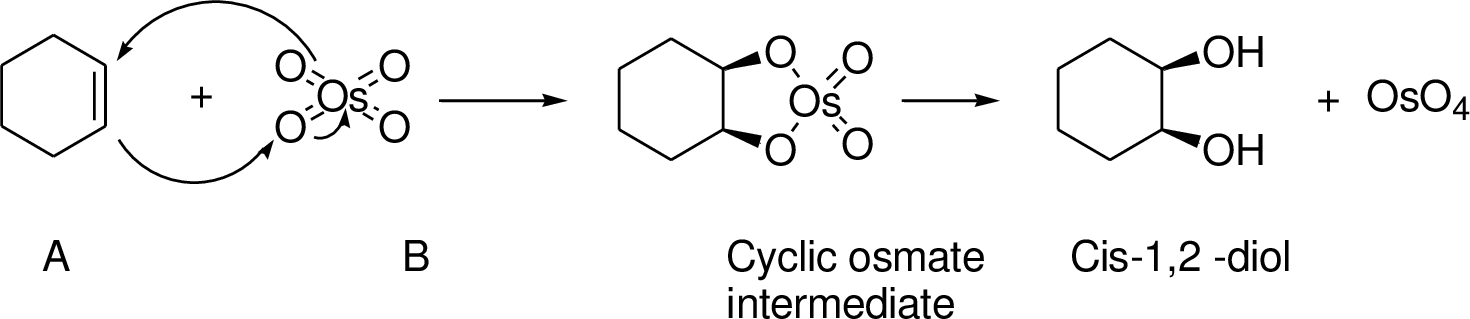
Explanation of Solution
The reaction is shown below,
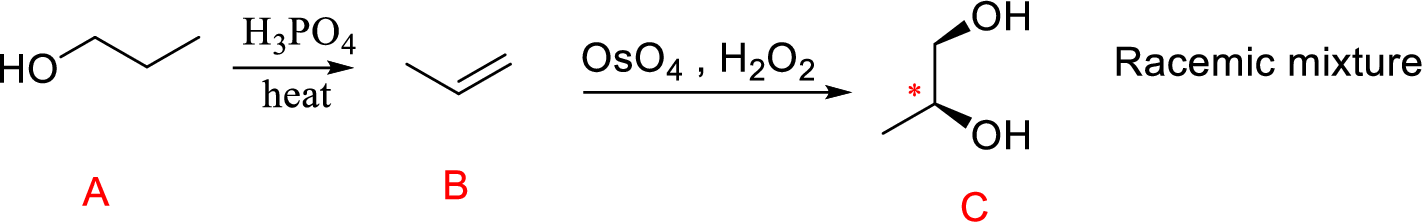
Acid-catalyzed dehydration gives the alkene compound (B) and the alkene (B) undergoes oxidation using osmium tetra oxide gives cyclic osmate intermediate, this intermediate further reaction with hydrogen peroxide hydrolysis that leads to product 1,2 diol (C). The racemic mixture obtained.
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry, Loose-leaf Version
Additional Science Textbook Solutions
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Biology: Life on Earth (11th Edition)
Organic Chemistry
Fundamentals Of Thermodynamics
Human Biology: Concepts and Current Issues (8th Edition)
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO2 (g) = N2O4(g) AGº = -5.4 kJ Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system: Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to '2' rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. 00 rise ☐ x10 fall yes no ☐ atm G Ar 1arrow_forwardWhy do we analyse salt?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H H CH3OH, H+ H Select to Add Arrows H° 0:0 'H + Q HH ■ Select to Add Arrows CH3OH, H* H. H CH3OH, H+ HH ■ Select to Add Arrows i Please select a drawing or reagent from the question areaarrow_forward
- What are examples of analytical methods that can be used to analyse salt in tomato sauce?arrow_forwardA common alkene starting material is shown below. Predict the major product for each reaction. Use a dash or wedge bond to indicate the relative stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts H Šali OH H OH Select to Edit Select to Draw 1. BH3-THF 1. Hg(OAc)2, H2O =U= 2. H2O2, NaOH 2. NaBH4, NaOH + Please select a drawing or reagent from the question areaarrow_forwardWhat is the MOHR titration & AOAC method? What is it and how does it work? How can it be used to quantify salt in a sample?arrow_forward
- Predict the major products of this reaction. Cl₂ hv ? Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. Note for advanced students: you can ignore any products of repeated addition. Explanation Check Click and drag to start drawing a structure. 80 10 m 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility DII A F1 F2 F3 F4 F5 F6 F7 F8 EO F11arrow_forwardGiven a system with an anodic overpotential, the variation of η as a function of current density- at low fields is linear.- at higher fields, it follows Tafel's law.Calculate the range of current densities for which the overpotential has the same value when calculated for both cases (the maximum relative difference will be 5%, compared to the behavior for higher fields).arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AGº = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of N will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Х ด ? olo 18 Ararrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

