
Without referring to your textbook or a periodic table, write the full electron configuration, the orbital box diagram, and the noble gas shorthand configuration for the elements with the following
msp;
(a)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 1.
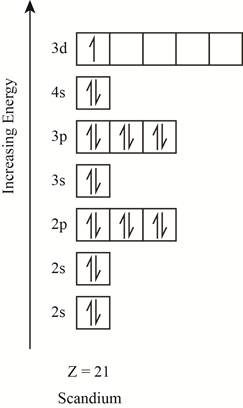
Figure 1
The electronic configuration of the given element can also be written as
(b)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 2.
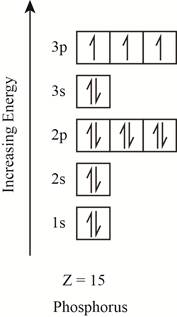
Figure 2
The electronic configuration of the given element can also be written as
(c)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 3.
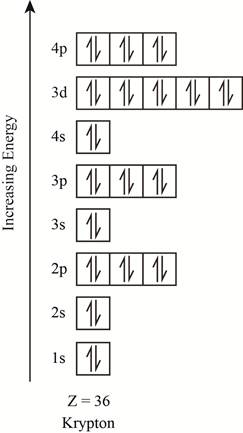
Figure 3
The electronic configuration of the given element can also be written as
(d)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 4.
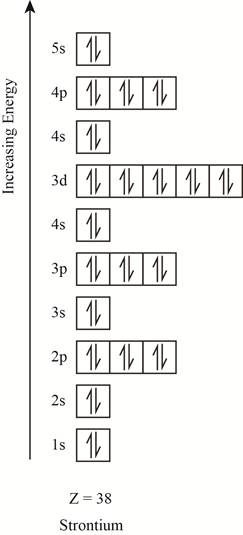
Figure 4
The electronic configuration of the given element can also be written as
(e)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 5.
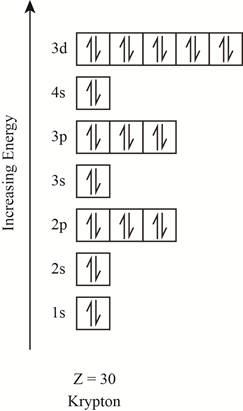
Figure 5
The electronic configuration of the given element can also be written as
Want to see more full solutions like this?
Chapter 11 Solutions
Introductory Chemistry
- Can you please explain to me this problem im very confused and lost. Help me step by step and in detail im soo lost.arrow_forward2) There are many forms of cancer, all of which involve abnormal cell growth. The growth and production of cells, called cell proliferation, is known to involve an enzyme called protein farnesyltransferase (PFTase). It is thought that inhibitors pf PFTase may be useful as anticancer drugs. The following molecule showed moderate activity as a potential PFTase inhibitor. Draw all stereoisomers of this compound. HO OHarrow_forwardConsidering rotation around the bond highlighted in red, draw the Newman projection for the most stable and least stable conformations when viewed down the red bond in the direction of the arrow. Part 1 of 2 H₁₂C H H Draw the Newman projection for the most stable conformation. Select a template to begin. Part 2 of 2 Draw the Newman projection for the least stable conformation. G 心arrow_forward
- personality of each of them in terms of nucleophile vs. electrophile (some can be considered acids/bases but we are not looking at that here). Note you may have to use your growing intuition to figure out the personality of one of the molecules below but I believe in you! Rationalize it out based on what we have called strong versus weak electrophiles in past mechanisms. Consider using the memes below to help guide your understanding! A OH O B CH3 C Molecule A: [Select] Molecule B: [Select] Molecule C: [Select] Molecule D: [Select] > H D OHarrow_forward4) Which oxygen atom in the structure below is most basic / nucleophilic? Please explain by discussing the electron density around each oxygen atom. Show at least three resonance structures for the compound. оогоarrow_forwardCan you show me this problem. Turn them into lewis dot structures for me please and then answer the question because I cant seem to comprehend it/ The diagrams on the picture look too small I guess.arrow_forward
- The fire releases 2.80 x 107 Joules of heat energy for each liter of oil burned. The water starts out at 24.5 °C, raising the water's temperature up to 100 °C, and then raises the temperature of the resulting steam up to 325 °C. How many liters of water will be needed to absorb the heat from the fire in this way, for each 1.0 liter of crude oil burned? 4186 J/(kg°C) = heat of water 2020 J/(kg°C) = heat of steam 2,256,000 (i.e. 2.256 x 106) J/kg = latent heat of vaporization for water (at the boiling point of 100 °C).arrow_forward6 Which of the following are likely to be significant resonance structures of a resonance hybrid? Draw another resonance structure for each of the compounds you select as being a resonance form. (A Br: Br: A B C D Earrow_forwardWrite the systematic (IUPAC) name for the following organic molecules. Note for advanced students: you do not need to include any E or Z prefixes in your names. Br structure Br Br Oweuarrow_forward
- Conservation of mass was discussed in the background. Describe how conservation of mass (actual, not theoretical) could be checked in the experiment performed.arrow_forwardWhat impact would adding twice as much Na2CO3 than required for stoichiometric quantities have on the quantity of product produced? Initial results attachedarrow_forwardGiven that a theoretical yield for isolating Calcium Carbonate in this experiment would be 100%. From that information and based on the results you obtained in this experiment, describe your success in the recovery of calcium carbonate and suggest two possible sources of error that would have caused you to not obtain 100% yield. Results are attached form experimentarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





