
Concept explainers
(a)
Interpretation:
The full electron configuration for the element with given
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electron configuration. The description of every electron in an orbital is given by the electron configuration of that atom.
Answer to Problem 96AP
The electron configuration of the given element with
Explanation of Solution
The electron configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is,
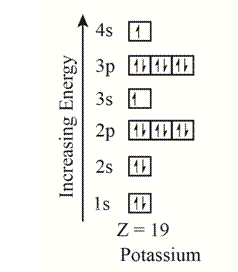
Figure 1
The electron configuration of the given element can also be written as
(b)
Interpretation:
The full electron configuration for the element with given atomic number, the orbital box diagram and the noble gas shorthand configuration ions are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electron configuration. The description of every electron that is moving freely in an orbital is given by the electron configuration of that atom.
Answer to Problem 96AP
The electron configuration of the given element with
Explanation of Solution
The electron configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is,
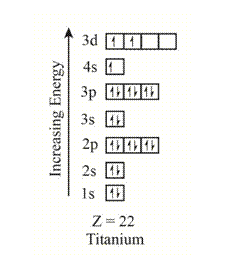
Figure 2
The electron configuration of the given element can also be written as
(c)
Interpretation:
The full electron configuration for the element with given atomic number, the orbital box diagram and the noble gas shorthand configuration ions are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electron configuration. The description of every electron in an orbital is given by the electron configuration of that atom.
Answer to Problem 96AP
The electron configuration of the given element with
Explanation of Solution
The electron configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is,
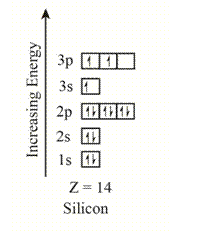
Figure 3
The electron configuration of the given element can also be written as
(d)
Interpretation:
The full electron configuration for the element with given atomic number, the orbital box diagram and the noble gas shorthand configuration ions are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electron configuration. The description of every electron in an orbital is given by the electron configuration of that atom.
Answer to Problem 96AP
The electron configuration of the given element with
Explanation of Solution
The electron configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is,
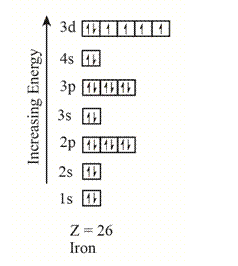
Figure 4
The electron configuration of the given element can also be written as
(e)
Interpretation:
The full electron configuration for the element with given atomic number, the orbital box diagram and the noble gas shorthand configuration ions are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electron configuration. The description of every electron in an orbital is given by the electron configuration of that atom.
Answer to Problem 96AP
The electron configuration of the given element with
Explanation of Solution
The electron configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is,
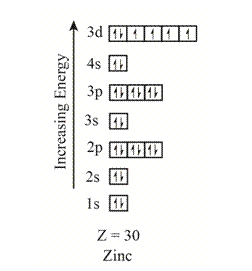
Figure 5
The electron configuration of the given element can also be written as
Want to see more full solutions like this?
Chapter 11 Solutions
Introductory Chemistry: A Foundation
- What are examples of analytical methods that can be used to analyse salt in tomato sauce?arrow_forwardA common alkene starting material is shown below. Predict the major product for each reaction. Use a dash or wedge bond to indicate the relative stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts H Šali OH H OH Select to Edit Select to Draw 1. BH3-THF 1. Hg(OAc)2, H2O =U= 2. H2O2, NaOH 2. NaBH4, NaOH + Please select a drawing or reagent from the question areaarrow_forwardWhat is the MOHR titration & AOAC method? What is it and how does it work? How can it be used to quantify salt in a sample?arrow_forward
- Predict the major products of this reaction. Cl₂ hv ? Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. Note for advanced students: you can ignore any products of repeated addition. Explanation Check Click and drag to start drawing a structure. 80 10 m 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility DII A F1 F2 F3 F4 F5 F6 F7 F8 EO F11arrow_forwardGiven a system with an anodic overpotential, the variation of η as a function of current density- at low fields is linear.- at higher fields, it follows Tafel's law.Calculate the range of current densities for which the overpotential has the same value when calculated for both cases (the maximum relative difference will be 5%, compared to the behavior for higher fields).arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AGº = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of N will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Х ด ? olo 18 Ararrow_forward
- Four liters of an aqueous solution containing 6.98 mg of acetic acid were prepared. At 25°C, the measured conductivity was 5.89x10-3 mS cm-1. Calculate the degree of dissociation of the acid and its ionization constant.Molecular weights: O (15.999), C (12.011), H (1.008).Limiting molar ionic conductivities (λ+0 and λ-0) of Ac-(aq) and H+(aq): 40.9 and 349.8 S cm-2 mol-1.arrow_forwardDetermine the change in Gibbs energy, entropy, and enthalpy at 25°C for the battery from which the data in the table were obtained.T (°C) 15 20 25 30 35Eo (mV) 227.13 224.38 221.87 219.37 216.59Data: n = 1, F = 96485 C mol–1arrow_forwardIndicate the correct options.1. The units of the transport number are Siemens per mole.2. The Siemens and the ohm are not equivalent.3. The Van't Hoff factor is dimensionless.4. Molar conductivity does not depend on the electrolyte concentration.arrow_forward
- Ideally nonpolarizable electrodes can1. participate as reducers in reactions.2. be formed only with hydrogen.3. participate as oxidizers in reactions.4. form open and closed electrochemical systems.arrow_forwardIndicate the options for an electrified interface:1. Temperature has no influence on it.2. Not all theories that describe it include a well-defined electrical double layer.3. Under favorable conditions, its differential capacitance can be determined with the help of experimental measurements.4. A component with high electronic conductivity is involved in its formation.arrow_forwardTo describe the structure of the interface, there are theories or models that can be distinguished by:1. calculation of the charge density.2. distribution of ions in the solution.3. experimentally measured potential difference.4. external Helmoltz plane.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





