
Study Guide for Chemistry: The Central Science
13th Edition
ISBN: 9780321949288
Author: Theodore E. Brown, James C. Hill
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 69E
Balance these equations by providing the missing coefficients
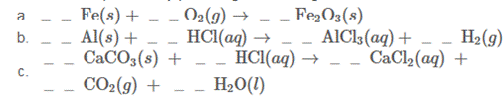
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please, help me out with the calculation, step by step on how to find what's blank with the given information.
Predict the following products. Then show the mechanism.
H₂N
NH2
BF3, Boron Trifluoride, known to contain three covalent boron-fluorine bonds. suggest and illustrate all of the processes as well as their energetical consequences for the formation of BF3 from its elements.
Chapter 11 Solutions
Study Guide for Chemistry: The Central Science
Ch. 11.2 - Only two isotopes of copper occur naturally:63Cu...Ch. 11.2 - 2.36 Rubidium has two naturally occurring...Ch. 11.3 - a. Thomson’s cathode-ray tube (Figure 2.49) and...Ch. 11.3 -
2.38 Consider the mass spectrometer shown in...Ch. 11.4 - Naturally occurring magnesium has the following...Ch. 11.4 - Mass spectrometry is more often applied to...Ch. 11.5 - 2-41 For each of the following elements, write its...Ch. 11.5 - Locate each of the following elements in the...Ch. 11.6 - 2-43 For each of the following elements, write its...Ch. 11.6 - 2.44 The elements of group 4A show an interesting...
Ch. 11.7 - 2.45 The structural formulas of the compounds...Ch. 11.7 - 2.46 Ball-and-stick representations of benzene, a...Ch. 11 - Prob. 1DECh. 11 - 2.59 Using the periodic table to guide you,...Ch. 11 -
2.71 Name the following ionic compounds:
a....Ch. 11 -
2.83
What is a functional group?
What functional...Ch. 11 - The element lead (Pb) consists of four naturally...Ch. 11 - Prob. 5ECh. 11 - The molecules have the same molecular formula...Ch. 11 - A sample of an ionic compound containing iron and...Ch. 11 -
The compound dioxane, which is used as a solvent...Ch. 11 - If 3.00 g of titanium metal is reacted with 6.00 g...Ch. 11 -
2.48 Two substances have the same molecular and...Ch. 11 - 2.49 Write the empirical formula corresponding to...Ch. 11 - Determine the molecular and empirical formulas of...Ch. 11 - 251 How many hydrogen atoms are un each of the...Ch. 11 - Prob. 14ECh. 11 - 253 Write the molecular and structural formulas...Ch. 11 - 2-54 Write the molecular and structural formulas...Ch. 11 - Fill in the gaps in the following table’Ch. 11 - 2.56 Fill in the gaps in the following...Ch. 11 - Prob. 19ECh. 11 - Prob. 20ECh. 11 - Predict the chemical formulas of the compounds...Ch. 11 - Prob. 22ECh. 11 - Prob. 23ECh. 11 - Predict whether each of the following compounds is...Ch. 11 - 2.66 Which of the following are ionic, and which...Ch. 11 - Prob. 26ECh. 11 - Prob. 27ECh. 11 -
2.69 Give the names and charges of the cation and...Ch. 11 - Give the names and charges of the cation and anion...Ch. 11 - Prob. 30ECh. 11 -
Give the chemical formula for each of the...Ch. 11 -
2.75 Give the name or chemical formula, as...Ch. 11 - Prob. 33ECh. 11 -
2.T Give the name or Chemical formula, as...Ch. 11 - Prob. 35ECh. 11 - Prob. 36ECh. 11 - Assume that you encounter the following sentences...Ch. 11 - a. What is a hydrocarbon? b. Pentane is the alkane...Ch. 11 - Prob. 39ECh. 11 -
2.85 Chloropropane is derived from propane by...Ch. 11 - Prob. 41ECh. 11 - Suppose a scientist repeats the Millikan oil-drop...Ch. 11 -
2.88 The natural abundance of 3He is...Ch. 11 - A cube of gold that is 1.00 cm on a side has a...Ch. 11 -
2.90 The diameter of a rubidium atom is 4.95 A....Ch. 11 -
2.91
Assuming the dimensions of the nucleus and...Ch. 11 - (a) What is the significance of the critical...Ch. 11 -
2.93 The nucleus of 6Li is a powerful absorber of...Ch. 11 - The element oxygen has three naturally occurring...Ch. 11 - Using a suitable reference such as the CRC...Ch. 11 - There are two different isotopes of bromine atoms....Ch. 11 -
2.99 It is common in mass spectrometry to assume...Ch. 11 - From the following list of elements—Ar, H, Ga, Al,...Ch. 11 - Prob. 54ECh. 11 -
2.102 The explosion of an atomic bomb releases...Ch. 11 - Prob. 56ECh. 11 - Prob. 57ECh. 11 -
2.105 From the molecular structures shown here,...Ch. 11 -
2.106 Name each of the following oxides. Assuming...Ch. 11 - Prob. 60ECh. 11 - Prob. 61ECh. 11 - Give the chemical names of each of the following...Ch. 11 -
2.112 Many familiar substances have common,...Ch. 11 -
2.113 Because many ions and compounds have very...Ch. 11 -
2.114 In what part of the atom does the strong...Ch. 11 - In the following diagram, the white spheres...Ch. 11 - In the following digram, the white spheres...Ch. 11 - Prob. 68ECh. 11 - Balance these equations by providing the missing...Ch. 11 - Write the balanced equation for the reaction that...Ch. 11 - Prob. 71ECh. 11 - Which of the following is the correct formula...Ch. 11 - Prob. 73AECh. 11 - Prob. 74AECh. 11 - Calculate the percentage of potassium by mass in...Ch. 11 - Which of the following samples contains the fewest...Ch. 11 - In dichloromethane, CH2Cl2 (= 1.60D)), the...Ch. 11 - Prob. 78AECh. 11 -
How many oxygen atoms are in (a) 0.25 mol...Ch. 11 - Prob. 80AECh. 11 - Prob. 81AECh. 11 - What is the mass, in grams, of 6.33 mol of NaHC03...Ch. 11 - What is the mass, in grams, of (a) 0.50 mol of...Ch. 11 - How many chlorine atoms are in 12.2 g of CCL4? a....Ch. 11 -
a. How many nitric acid molecules are in 4.20 g...Ch. 11 - A 2.144-g sample of phosgene, a compound used as a...Ch. 11 - A 5.325-g sample of methyl benzoate, a compound...Ch. 11 -
Cyclohexane a commonly used organic solvent, is...Ch. 11 - Prob. 89AECh. 11 - Prob. 90IECh. 11 - Decomposition of KCIO3 is sometimes used to...Ch. 11 - Propane, C3 H8 (Figure 3.8), is a common fuel used...Ch. 11 -
Methanol, CH3OH, reacts with oxygen from air in a...Ch. 11 - When 24 mol of methanol and 15 mol of oxygen...Ch. 11 - a. When 1.50 mol of Al and 3.00 mol of Cl2 combine...Ch. 11 - Molten gallium reacts with arsenic to form the...Ch. 11 -
When a 2.00-g strip of zinc metal is placed in...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the mechanism of the reaction.arrow_forward9. Draw all of the possible Monochlorination Products that would Result From the Free Radical Chlormation OF 23,4-TRIMethyl Pentane b. Calculate the To Yield For the major • Product given the Following Relative Restritus For 1° 2° and 30 Hydrogens toward Free Radical Chloration 5.0: 38 : 1 30 2° 1° C. what would be the major product in the Free Radical brominator Of the Same Molecule. Explain your Reasoning.arrow_forwardWhat is the complete reaction mechanism for the chlorination of Ethane, C2H6?arrow_forward
- A 13C NMR spectrum is shown for a molecule with the molecular formula of C6H100. Draw the structure that best fits this data. 220 200 180 160 140 120100 80 60 40 20 Drawingarrow_forwardPlease help me figure out the blan areas with step by step calculations.arrow_forwardneeding help draw all of the possible monochlorination products that would result from the free radical chlorination of 2,3,4-trimethylpentanearrow_forward
- HAND DRAWarrow_forwardBased on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10. Provide assignment for the provided structurearrow_forwardO Predict the 'H NMR integration ratio for the following structure. IV I. 3 H A II. 1 H III. 2 H IV. 3 H I. 3 H B II. O H III. 2 H IV. 3 H I. 3 H C II. 2 H III. 2 Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY