
Concept explainers
(a)
Interpretation: The IUPAC name for the given cycloalkane needs to be determined.
Concept Introduction: According to the IUPAC rule,
(a)
Explanation of Solution
The given structure is as follows:
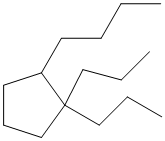
The above structure contains a cycloalkane ring with 5 carbon atoms thus, it is cyclopentane.
Here, numbering will be done according to the number of substituents present of cyclopentane ring. There are 2 substituent at one position and 1 substituent at other.
The numbering can be done as follows:
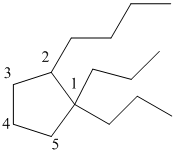
From the above structure, there are 2 propyl groups at 1st position and 1 butyl group at 2nd position of cyclopentane ring.
Thus, the IUPAC name of the molecule will be:
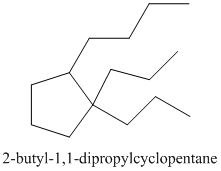
(b)
Interpretation: The IUPAC name for the given
Concept Introduction: According to the IUPAC rule, numbering of atom is done to find the longest continuous carbon chain in the molecule. After that name of groups attached to the chain are identified. The location of substituent groups is designated by numbers and name. The last step is to assemble the name by listing the groups in alphabetical order. Prefixes such as di, tri, tetra etc. are used to represent groups of same kind.
(b)
Explanation of Solution
The given structure is as follows:
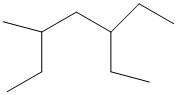
The numbering will be done according to the longest carbon chain in the molecule.
The numbering is done as follows:
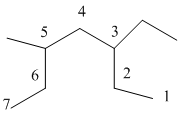
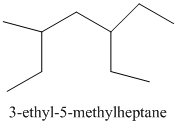
From the above structure, there is 1 ethyl group at 3rd position and 1 methyl group at 5th position of the main chain containing 7 carbon atoms.
Therefore, the IUPAC name of the structure will be:
Thus,
(c)
Interpretation: The IUPAC name for the given alkane needs to be determined.
Concept Introduction: According to the IUPAC rule, numbering of atom is done to find the longest continuous carbon chain in the molecule. After that name of groups attached to the chain are identified. The location of substituent groups is designated by numbers and name. The last step is to assemble the name by listing the groups in alphabetical order. Prefixes such as di, tri, tetra etc. are used to represent groups of same kind.
(c)
Explanation of Solution
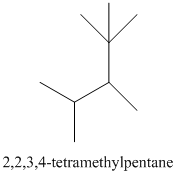
The given structure is as follows:
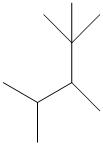
The numbering will be done according to the longest carbon chain in the molecule.
The numbering can be done as follows:
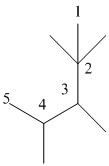
Thus, there are 5 carbon atoms in the parent chain. Also, there are 2 methyl groups at 2nd position, 1 methyl group at 3rd position and 1 methyl group at 4th position. There are total 4 methyl substituents.
Therefore, the IUPAC name of the molecule will be:
(d)
Interpretation: The IUPAC name for the given cycloalkane needs to be determined.
Concept Introduction: According to the IUPAC rule, numbering of atom is done to find the longest continuous carbon chain in the molecule. After that name of groups attached to the chain are identified. The location of substituent groups is designated by numbers and name. The last step is to assemble the name by listing the groups in alphabetical order. Prefixes such as di, tri, tetra etc. are used to represent groups of same kind.
(d)
Explanation of Solution
The given structure is as follows:
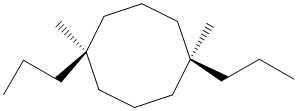
It contains a cycloalkane with 8 carbon atoms. Two substituents are same thus, numbering will start from any one of the substituent side.
The numbering can be represented as follows:
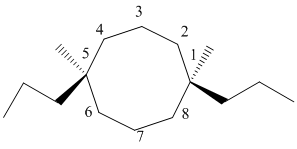
From the above structure, there is 1 methyl and 1 propyl group at 1st and 6th position.
Thus, IUPAC name will be as follows:
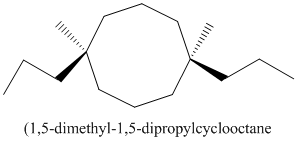
Stereochemistry can also be determined by giving priority to the groups attached.
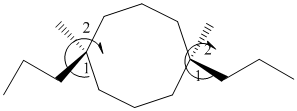
The 1-2 movement is clockwise but the lowest priority group is in front of the curved arrow so assignment will be reversed. Here, clockwise is S and anti or counterclockwise is R. Since, in the above structure the movement is clockwise so the configuration will be S for both sides. The IUPAC name with configuration will be:
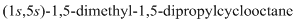
(e)
Interpretation: The IUPAC name for the given cycloalkane needs to be determined.
Concept Introduction: According to the IUPAC rule, numbering of atom is done to find the longest continuous carbon chain in the molecule. After that name of groups attached to the chain are identified. The location of substituent groups is designated by numbers and name. The last step is to assemble the name by listing the groups in alphabetical order. Prefixes such as di, tri, tetra etc. are used to represent groups of same kind.
(e)
Explanation of Solution
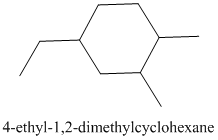
The given structure is as follows:
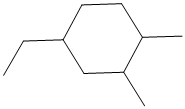
It contains a cycloalkane ring with 6 carbon atoms.
The numbering is represented as follows:
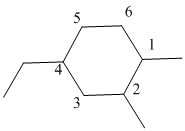
From the above structure, there is 1 methyl group at 1st position, 1 methyl group at 2nd position and 1 ethyl group at 4th position.
Thus, IUPAC name of the molecule will be:
(f)
Interpretation: The IUPAC name for the given alkane needs to be determined.
Concept Introduction: According to the IUPAC rule, numbering of atom is done to find the longest continuous carbon chain in the molecule. After that name of groups attached to the chain are identified. The location of substituent groups is designated by numbers and name. The last step is to assemble the name by listing the groups in alphabetical order. Prefixes such as di, tri, tetra etc. are used to represent groups of same kind.
(f)
Explanation of Solution
The given structure is as follows:
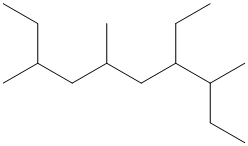
The numbering will be done according to the longest carbon chain in the molecule.
The numbering can be done as follows:
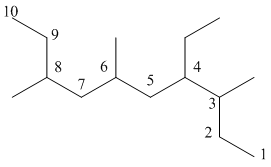
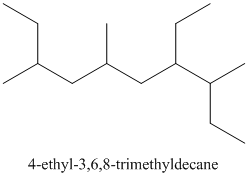
From the above structure, there is 1 ethyl group at 4th position and 1 methyl group each at 3rd, 6th and 8th carbon atom in the chain.
Thus, the IUPAC name of the molecule will be:
Want to see more full solutions like this?
Chapter 11 Solutions
INTRO.TO GENERAL,ORGAN...-OWLV2 ACCESS
- molecule 0= OH ☐ ☐ type of molecule (check all that apply) fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated ☐ polyunsaturated ☐ ☐ ☐ ☐ ☐ 010 0 0 0 0 0 0 ☐ ☐ ☐ ☐☐☐☐ U omega-3 omega-6 fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated omega-3 omega-6 fatty acid monoglyceride diglyceride triglyceride saturated unsaturated monounsaturated polyunsaturated omega-3 omega-6 OH OHarrow_forward'☐ : ☑ ด Suppose an alien life form has DNA just like human DNA remain the same.) - except that the alien DNA is made from deoxyarabinose instead of deoxyribose. (All other ingredients Draw the structure of a nucleotide containing thymine from which the alien DNA would be assembled. Note: be sure to draw the molecule as it would exist at physiological pH. Click and drag to start drawing a structure.arrow_forwardPredict the products of the following biochemical reaction: CH2 CH-O + 3 KOH CH2-0 In particular, draw the structure of the product or products P in the drawing area below. If there are no products, because this reaction won't happen, check the No reaction box under the drawing area. Note: if there is more than one product, you can draw them in any arrangement you like. Also, just draw the structure of each product. You don't have to draw the complete right-hand side of the equation, including stoichiometric coefficients. No reaction Click and drag to start drawing a structure. : 5 èarrow_forward
- Name 1) 3-fluoro, 1-butene 2) 2-heptene 2,3-difluoro- 1-pentene 4) 6-iodo,4-methyl- 2-decyne 5) 4,4-dibromo- 1,2-butandiol Complete structural formula F -C=C-C-C- Line formula Condensed structural formula N F CH2=CHCHFCH3arrow_forward1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forward
- Under aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardAssign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





