
CHEMISTRY+CHEM...(LL)-W/ACCESS >CUSTOM<
10th Edition
ISBN: 9780357096949
Author: Kotz
Publisher: CENGAGE C
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 40IL
A “hand boiler” can be purchased in toy stores or at science supply companies. If you cup your hand around the bottom bulb, the volatile liquid in the boiler boils, and the liquid moves to the upper chamber.
- (a) Using your knowledge of kinetic molecular theory and intermolecular forces, explain how the hand boiler works.

- (b) Which of the following liquids would be best to use in the hand boiler? Explain.
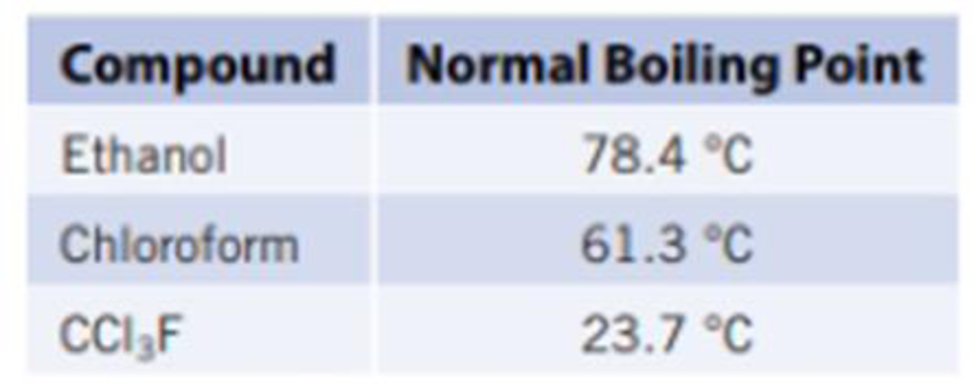
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C.
H₂O(g) + C₁₂O(g) = 2 HOCl(g)
K = 0.0900 at 25°C
с
Calculate the equilibrium concentrations of each gas at 25 °C.
[H₂O]=
[C₁₂O]=
[HOCI]=
M
Σ
M
What units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?
Provide the structure, circle or draw, of the monomeric unit found in the biological polymeric
materials given below.
HO
OH
amylose
OH
OH
행
3
HO
cellulose
OH
OH
OH
Ho
HO
Chapter 11 Solutions
CHEMISTRY+CHEM...(LL)-W/ACCESS >CUSTOM<
Ch. 11.2 - Which should have the more negative hydration...Ch. 11.3 - Using structural formulas, describe the hydrogen...Ch. 11.4 - Prob. 11.3CYUCh. 11.5 - Prob. 11.4CYUCh. 11.6 - The molar enthalpy of vaporization of methanol,...Ch. 11.6 - Prob. 11.6CYUCh. 11.6 - Prob. 1.1ACPCh. 11.6 - Prob. 1.2ACPCh. 11.6 - Prob. 2.1ACPCh. 11.6 - Prob. 2.2ACP
Ch. 11 - Prob. 1PSCh. 11 - Intermolecular forces: What type of forces must be...Ch. 11 - Prob. 3PSCh. 11 - Prob. 4PSCh. 11 - Considering intermolecular forces in the pure...Ch. 11 - Considering intermolecular forces in the pure...Ch. 11 - Prob. 7PSCh. 11 - Which of the following compounds would be expected...Ch. 11 - Prob. 9PSCh. 11 - When salts of Mg2+, Na+, and Cs+ are placed in...Ch. 11 - Prob. 11PSCh. 11 - The enthalpy of vaporization of liquid mercury is...Ch. 11 - Answer the following questions using Figure 11.12:...Ch. 11 - Answer the following questions using Figure 11.12:...Ch. 11 - Prob. 15PSCh. 11 - Refer to Figure 11.12 to answer these questions:...Ch. 11 - Which member of each of the following pairs of...Ch. 11 - Place the following four compounds in order of...Ch. 11 - Prob. 19PSCh. 11 - You are comparing three different substances, A,...Ch. 11 - Equilibrium vapor pressures of benzene, C6H6, at...Ch. 11 - Prob. 22PSCh. 11 - Can carbon monoxide (Tc = 132.9 K; Pc = 34.5 atm...Ch. 11 - Methane (CH4) cannot be liquefied at room...Ch. 11 - What is surface tension? Give an example...Ch. 11 - What factors affect the viscosity of a substance?...Ch. 11 - If a piece of filter paper (an absorbent paper...Ch. 11 - When water is placed in a buret it forms a concave...Ch. 11 - Prob. 29GQCh. 11 - What types of intermolecular forces are important...Ch. 11 - Which of the following salts, Li2SO4 or Cs2SO4, is...Ch. 11 - Prob. 32GQCh. 11 - Prob. 33GQCh. 11 - Prob. 34GQCh. 11 - Rank the following compounds in order of...Ch. 11 - Prob. 36GQCh. 11 - Prob. 37GQCh. 11 - The following data are the equilibrium vapor...Ch. 11 - Prob. 39ILCh. 11 - A hand boiler can be purchased in toy stores or at...Ch. 11 - Prob. 41ILCh. 11 - Prob. 42ILCh. 11 - Acetone, CH3COCH3, is a common laboratory solvent....Ch. 11 - Cooking oil floats on top of water. From this...Ch. 11 - Liquid ethylene glycol, HOCH2CH2OH, is one of the...Ch. 11 - Liquid methanol, CH3OH, is placed in a glass tube....Ch. 11 - Account for these facts: (a) Although ethanol...Ch. 11 - Prob. 48SCQCh. 11 - Prob. 49SCQCh. 11 - Prob. 50SCQCh. 11 - Prob. 51SCQCh. 11 - Prob. 52SCQCh. 11 - A fluorocarbon, CF4, has a critical temperature of...Ch. 11 - Prob. 55SCQCh. 11 - List four properties of liquids that are directly...Ch. 11 - List the following ions in order of hydration...Ch. 11 - Prob. 59SCQCh. 11 - An 8.82-g sample of Br2 is placed in an evacuated...Ch. 11 - Polarizability is defined as the extent to which...Ch. 11 - Prob. 62SCQCh. 11 - A pressure cooker (a kitchen appliance) is a pot...Ch. 11 - Vapor pressures of NH3() at several temperatures...Ch. 11 - Prob. 65SCQCh. 11 - Prob. 66SCQCh. 11 - Prob. 67SCQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- OA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forwardDon't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forward
- Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forwardClassify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forwardQ1: Rank the relative nucleophilicity of the following species in ethanol. CH3O¯, CH3OH, CH3COO, CH3COOH, CH3S Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Viscosity, Cohesive and Adhesive Forces, Surface Tension, and Capillary Action; Author: Professor Dave Explains;https://www.youtube.com/watch?v=P_jQ1B9UwpU;License: Standard YouTube License, CC-BY