
Concept explainers
Write structural formulas for each of the following:
Interpretation:
The structural formula of the given compounds is to be written.
Concept introduction:
When writing the structural formula of any compound, first the functional group from the suffix of the given name is identified.
The longest carbon chain containing the functional group is located.
The carbon atoms of the chain are numbered in a way that the functional group is at lowest numbered carbon atom.
Substituents are attached to the parent chain according to their positions given in the name.
In alkenes, the Z isomers have the higher ranked substituents on the same side of the double bond, and in E isomers, higher ranked substituents are on the opposite sides of the double bond.
Answer to Problem 26P
Solution:
a)
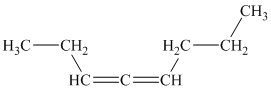
b)
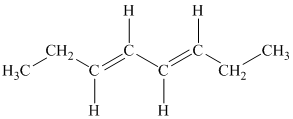
c)
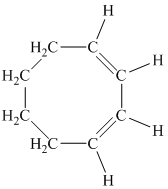
d)
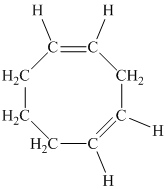
e)
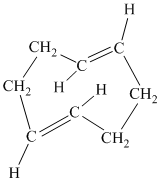
f)
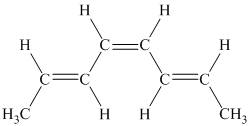
g)
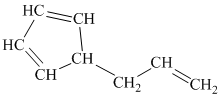
h)
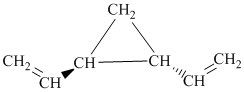
i)
Explanation of Solution
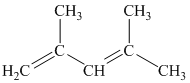
a) The given compound is
The parent name contains the word “
The structural formula is as follows:

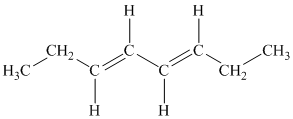
b) The given compound is
The parent name contains the word “
The structural formula is as follows:
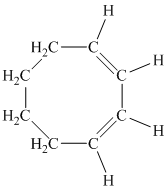
c) The given compound is
The parent name contains the word “cycloocta” in it, which means that the parent carbon chain contains eight carbon atoms in a cyclic form. The suffix added is
The structural formula is as follows:
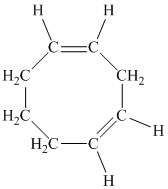
d) The given compound is
The parent name contains the word “cycloocta” in it, which means that the parent carbon chain contains eight carbon atoms in a cyclic form. The suffix added is
The structural formula is as follows:
e) The given compound is
The parent name contains the word “cycloocta” in it, which means that the parent carbon chain contains eight carbon atoms in a cyclic form. The suffix added is
The structural formula is as follows:
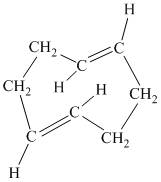
f) The given compound is
The parent name contains the word “
The structural formula is as follows:
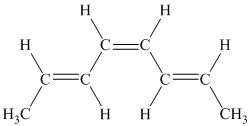
g) The given compound is
The parent name contains the word “cyclopenta” in it, which means that the parent carbon chain contains five carbon atoms in a cyclic form. The suffix added is
The structural formula is as follows:
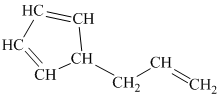
h) The given compound is
The parent name contains the word cyclopropane in it, which means that the parent carbon chain contains three carbon atoms in a cyclic form. There are two vinyl groups attached to the cyclopropane. The numbers
The structural formula is as follows:
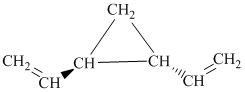
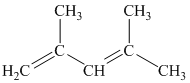
i) The given compound is
The parent name contains the word “penta” in it, which means that the longest carbon chain contains five carbon atoms. The suffix added is
The structural formula is as follows:
Want to see more full solutions like this?
Chapter 11 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- ion. A student proposes the following Lewis structure for the perchlorate (CIO) io : :0: : Cl : - - : :0: ك Assign a formal charge to each atom in the student's Lewis structure. atom central O formal charge ☐ top O ☐ right O ☐ bottom O ☐ Cl ☐arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Yes. Is the proposed Lewis structure reasonable? Cl- : 2: :Z: :Z: N—N : 0: C C1: O CO No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". ☑arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A This reaction is faster above 103. °C than below. AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only above -9. °C. AS is (pick one) ΔΗ is (pick one) C The reverse of this reaction is always spontaneous. AS is (pick one) 18 Ararrow_forward
- Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forwardplease explain this in simple termsarrow_forward
- K Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward(2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forwardDraw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forward
- Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forwardDraw the Lewis structure for the polyatomic trisulfide anion. Be sure to include all resonance structures that satisfy the octet rule. с [ ] - Garrow_forward1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on the LC-MS printout. How much different are they? 2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit, explain what each of these is and why they are present. 3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by calculating the accurate monoisotopic mass. 4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source. 5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one point of extra credit, see if you can identify this molecule as well and confirm by…arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

