
Equilibrium vapor pressures of benzene, C6H6, at various temperatures are given in the table.
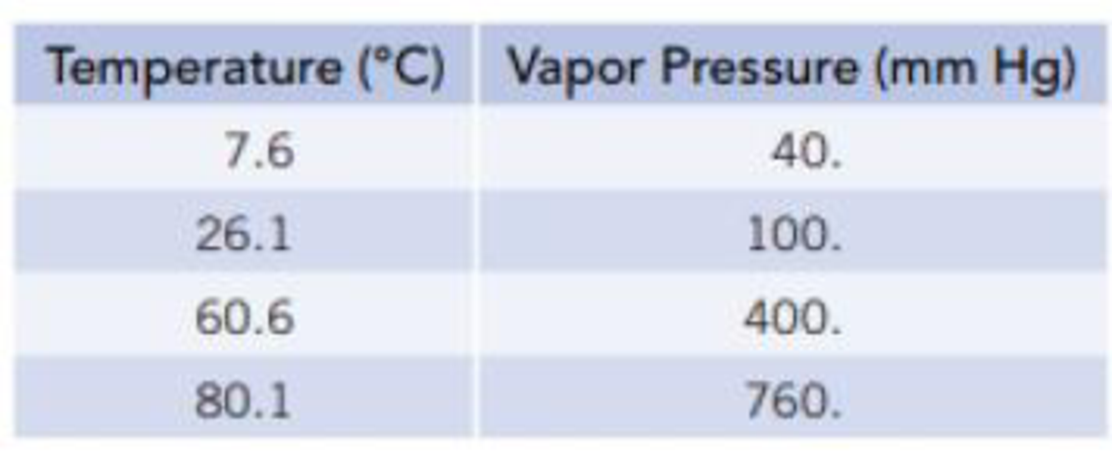
- (a) What is the normal boiling point of benzene?
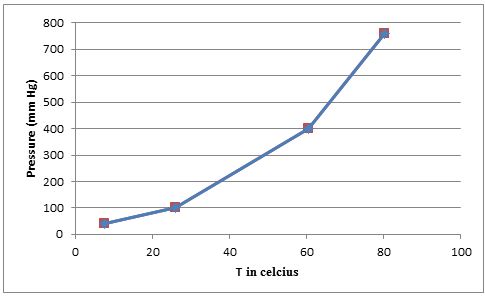
- (b) Plot these data so that you have a graph resembling the one in Figure 11.12. At what temperature does the liquid have an equilibrium vapor pressure of 250 mm Hg? At what temperature is the vapor pressure 650 mm Hg?
- (c) Calculate the molar enthalpy of vaporization for benzene using the Clausius–Clapeyron equation.
(a)
Interpretation:
The normal boiling point of benzene has to be determined.
Concept Introduction:
Boiling point: It is the temperature at which liquid converts to vapor. At boiling point the vapor pressure of liquid and the pressure of the surroundings are equal.
Normal boiling point: When the external pressure is
Answer to Problem 21PS
The normal boiling point of benzene is
Explanation of Solution
The normal boiling point of benzene is calculated
Given:
Normal boiling point is the temperature when the external pressure is
From the given data it is clear that the temperature at which the pressure is
Thus the normal boiling point of benzene is
(b)
Interpretation:
The temperature versus vapor pressure graph should be plotted. The temperatures at which the liquid has vapour pressures of
Concept Introduction:
Vapor pressure is nothing but the pressure of a vapor in contact with its liquid or solid form.
When a liquid and vapor are in equilibrium the pressure exerted by the vapor is called the equilibrium vapor pressure
Answer to Problem 21PS
The temperatures at which liquid have a vapour pressures of
Explanation of Solution
Given,
The temperatures at which liquid have a vapour pressures of
Using the given data we can plot the graph of
From the graph we can find the approximate temperatures at which the pressures are
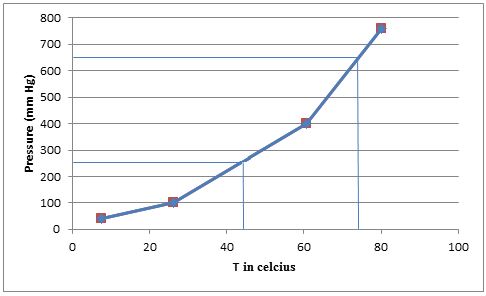
Therefore,
The temperature at which the pressure is
(c)
Interpretation:
The molar enthalpy of vaporization using Clausius-Clapeyron has to be determined
Concept Introduction:
Clausius-Clapeyron equation:
From this relationship we can calculate the molar enthalpy of vaporization by knowing the corresponding temperature and pressure values.
If we have pressures at two different temperatures, then enthalpy of vaporization can be calculated by
Answer to Problem 21PS
The molar enthalpy of vaporization of is
Explanation of Solution
The molar enthalpy of vaporization is calculated using the given data,
Given:
Clausius-Clapeyron equation is,
Substituting the values
The molar enthalpy of vaporization of is
Want to see more full solutions like this?
Chapter 11 Solutions
CHEMISTRY+CHEM...HYBRID ED.(LL)>CUSTOM<
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Organic Chemistry
Loose Leaf For Integrated Principles Of Zoology
Chemistry: Structure and Properties (2nd Edition)
- Q3: Write in the starting alkyl bromide used to form the following products. Include any reactants, reagents, and solvents over the reaction arrow. If more than one step is required, denote separate steps by using 1), 2), 3), etc. H OH racemic OH OH 5 racemicarrow_forwardDraw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.arrow_forwardPredict all organic product(s), including stereoisomers when applicable.arrow_forward
- What I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forwardConsider the solubility products (Ksp values) for the following compounds:SrSO4 (Ksp = 7.6 x 10−7), BaSO4 (Ksp = 1.5 x 10−9), SrCO3 (Ksp = 7.0 x 10−10), BaCO3 (Ksp = 1.6 x 10−9)Which anion is the harder base, CO32− or SO42−? Justify your answer.arrow_forwardQ1: a) Arrange the compounds in order of decreasing pKa, highest first. ОН ΟΗ ῸΗ дон ОН ОН CI Brarrow_forward
- (4 pts - 2 pts each part) A route that can be taken to prepare a hydrophobic (water-repellent) aerogel is to start with trichloromethylsilane, CH3SiCl3 as the silicon source. a. What is the chemical reaction that this undergoes to form a product with Si-OH groups? Write as complete of a chemical equation as you can. CI CI-SI-CH3 CI b. The formation of a byproduct is what drives this reaction - what is the byproduct (if you didn't already answer it in part (a)) and how/why does it form?arrow_forwardb) Circle the substrate that would not efficiently generate a Grignard reagent upon reaction with Mg in ether. CI Br ד c) Circle the Grignard reagents that contain incompatible functional groups. MgBr HO MgBr MgBr MgBr MgBr HO MgBrarrow_forwardQ2: Predict all organic product(s), including stereoisomers when applicable. PCC OH a) CH2Cl2 Page 2 of 5 Chem 0310 Organic Chemistry 1 HW Problem Sets b) .OH Na2Cr2O7, H+ OH PCC CH2Cl2 c) OHarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





