
Chemistry (OER)
19th Edition
ISBN: 9781947172623
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 19E
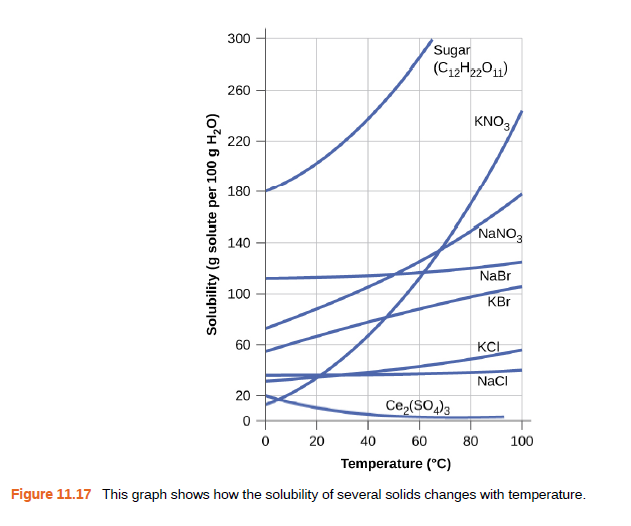
Calculate the percent by mass of KBr in a saturated solution of KBr in water at 10 °C. See Figure 11.17 for useful data, and report the computed percentage to one significant digit.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
PLEASE HELP! URGENT!
"Water gas" is an industrial fuel composed of a mixture of carbon monoxide and hydrogen gases. When this
fuel is burned, carbon dioxide and water result. From the information given below, write a balanced equation
and determine the enthalpy of this reaction:
CO(g) + O2(g) → CO₂(g) + 282.8 kJ
H2(g) + O2(g) → H₂O(g) + 241.8 kJ
MacBook Air
Page of 3
4. Calculate AG for the following reaction at 25°C. Will the reaction occur (be spontaneous)? How do you
know?
NH3(g) + HCl(g) → NH4Cl(s)
AH=-176.0 kJ
AS-284.8 J-K-1
Chapter 11 Solutions
Chemistry (OER)
Ch. 11 - How do solutions differ from compounds? From other...Ch. 11 - Which of the principal characteristics of...Ch. 11 - When KNO3 is dissolved in water, the resulting...Ch. 11 - Give an example of each of the following types of...Ch. 11 - Indicate the most important types of...Ch. 11 - Predict whether each of the following substances...Ch. 11 - Heat is released when some solutions form; heat is...Ch. 11 - Solutions of hydrogen in palladium may be formed...Ch. 11 - Explain why the ions Na+ and CI- are strongly...Ch. 11 - Explain why solutions of HBr in benzene (a...
Ch. 11 - Consider the solutions presented: (a) Which of the...Ch. 11 - Compare the processes that occur when methanol...Ch. 11 - What is the expected electrical conductivity of...Ch. 11 - Why are most solid ionic compounds electrically...Ch. 11 - Indicate the most important type of intermolecular...Ch. 11 - Suppose you are presented with a clear solution of...Ch. 11 - Supersaturated solutions of most solids in water...Ch. 11 - Suggest an explanation for the observations that...Ch. 11 - Calculate the percent by mass of KBr in a...Ch. 11 - Which of the following gases is expected to be...Ch. 11 - At 0 C and 1.00 atm, as much as 0.70 g of O2 can...Ch. 11 - Refer to Figure 11.11. (a) How did the...Ch. 11 - The Henry's law constant for CO2 is 3.4102 M/atm...Ch. 11 - The Henry's law constant for O2 is 1.3103M /atm at...Ch. 11 - How many liters of HCI gas, measured at 30.0 C and...Ch. 11 - Which is are part of the macroscopic domain of...Ch. 11 - What is the microscopic explanation for the...Ch. 11 - Sketch a qualitative graph of the pressure versus...Ch. 11 - A solution of potassium nitrate, an electrolyte,...Ch. 11 - What are the mole fractions of H3PO4 and water in...Ch. 11 - What are the mole fractions of HNO3 and water in a...Ch. 11 - Calculate the mole fraction of each solute and...Ch. 11 - Calculate the mole fraction of each solute and...Ch. 11 - Calculate the mole fractions of methanol, CH3OH;...Ch. 11 - What is the difference between a 1 M solution and...Ch. 11 - What is the molality of phosphoric acid, H3PO4, in...Ch. 11 - What is the molality of nitric acid in a...Ch. 11 - Calculate the molality of each of the following...Ch. 11 - Calculate the molality of each of the following...Ch. 11 - The concentration of glucose, C6H12O6, in normal...Ch. 11 - A 13.0% solution of K2CO3 by mass has a density of...Ch. 11 - Why does 1 mol of sodium chloride depress the...Ch. 11 - What is the boiling point of a solution of 115.0 g...Ch. 11 - What is the boiling point of a solution of 9.04 g...Ch. 11 - What is the freezing temperature of a solution of...Ch. 11 - What is the freezing point of a solution of 9.04 g...Ch. 11 - What is the osmotic pressure of an aqueous...Ch. 11 - What is osmotic pressure of a solution of bovine...Ch. 11 - What is the molar mass of a solution of 5.00 g of...Ch. 11 - A sample of an organic compound (a nonelectrolyte)...Ch. 11 - A 1.0 m solution of HCI in benzene has a freezing...Ch. 11 - A solution contains 5.00 g of urea, CO(NH2)2, a...Ch. 11 - A 12.0-g sample of a nonelectrolyte is dissolved...Ch. 11 - Arrange the following solutions in order by their...Ch. 11 - Calculate the boiling point elevation of 0.100 kg...Ch. 11 - How could you prepare a 3.08 m aqueous solution of...Ch. 11 - A sample of sulfur weighing 0.210 g was dissolved...Ch. 11 - In a significant experiment performed many years...Ch. 11 - Lysozyme is an enzyme that cleaves cell walls. A...Ch. 11 - The osmotic pressure of a solution containing 7.0...Ch. 11 - The osmotic pressure of human blood is 7.6 atm at...Ch. 11 - What is the freezing point of a solution of...Ch. 11 - What is the boiling point of a solution of NaCI in...Ch. 11 - The sugar fructose contains 40.0% C, 6.7% H, and...Ch. 11 - The vapor pressure of methanol, CH3OH, is 94 torr...Ch. 11 - The triple point of air-free water is defined as...Ch. 11 - Meat can be classified as fresh (not frozen) even...Ch. 11 - An organic compound has a composition of 93.46% C...Ch. 11 - A sample of HgCI2 weighing 9.41 g is dissolved in...Ch. 11 - A salt is known to be an alkali metal fluoride. A...Ch. 11 - Identify the dispersed phase and the dispersion...Ch. 11 - Distinguish between dispersion methods and...Ch. 11 - How do colloids differ from solutions with regard...Ch. 11 - Explain the cleansing action of soap.Ch. 11 - How can it be demonstrated that colloidal...
Additional Science Textbook Solutions
Find more solutions based on key concepts
58. Write full electron configurations and indicate the valence electrons and the core electrons for each eleme...
Introductory Chemistry (6th Edition)
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
4. Three groups of nonvascular plants are _______, ______, and _______. Three groups of seedless vascular plant...
Biology: Life on Earth (11th Edition)
1. ___ Mitosis 2. ___ Meiosis 3. __ Homologous chromosomes 4. __ Crossing over 5. __ Cytokinesis A. Cytoplasmic...
Microbiology with Diseases by Body System (5th Edition)
1. A person gets in an elevator on the ground floor and rides it to the top floor of a building. Sketch a veloc...
College Physics: A Strategic Approach (3rd Edition)
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Similar questions
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 5. 4NO2(g) ⇔ 2N2O4(g)arrow_forwardtrue or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forward
- True or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forwardtrue or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forward
- Which of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forwardPredict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forwardA 8.25 g sample of aluminum at 55°C released 2500 J of heat. The specific heat of aluminum is 0.900 J/g°C. The density of aluminum is 2.70 g/mL. Calculate the final temperature of the aluminum sample in °C.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning  General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning