
Chemistry
12th Edition
ISBN: 9780078021510
Author: Raymond Chang Dr., Kenneth Goldsby Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 11.92QP
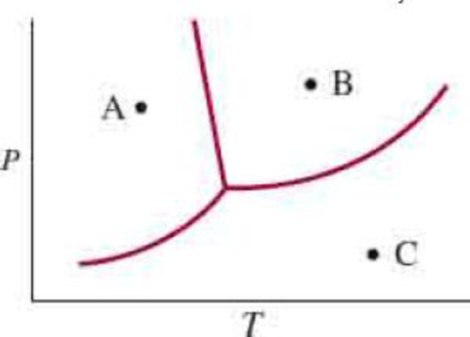
A phase diagram of water is shown at the end of this problem. Label the regions. Predict what would happen as a result of the following changes: (a) Starting at A, we raise the temperature at constant pressure. (b) Starting at C, we lower the temperature at constant pressure. (c) Starting at B, we lower the pressure at constant temperature.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate
the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data:
molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.
If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate
the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data:
molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.
Determine the distance between the metal and the OHP layer using the Helm-
holtz model when the electrode's differential capacitance is 145 μF cm².
DATA: dielectric constant of the medium for the interfacial zone &r=
lectric constant of the vacuum &0 = 8.85-10-12 F m-1
= 50, die-
Chapter 11 Solutions
Chemistry
Ch. 11.2 - Name the type(s) of intermolecular forces that...Ch. 11.2 - Prob. 2PECh. 11.2 - Which of the following compounds is most likely to...Ch. 11.3 - Why are motorists advised to use more viscous oils...Ch. 11.4 - When silver crystallizes, it forms face-centered...Ch. 11.4 - Tungsten crystallizes in a body-centered cubic...Ch. 11.5 - X rays of wavelength 0.154 nm are diffracted from...Ch. 11.5 - Prob. 1RCCh. 11.6 - Prob. 5PECh. 11.6 - Copper crystallizes in a face-centered cubic...
Ch. 11.6 - Prob. 1RCCh. 11.8 - Prob. 7PECh. 11.8 - Prob. 1RCCh. 11.8 - Calculate the heat released when 68.0 g of steam...Ch. 11.9 - Which phase diagram (a)(c) corresponds to a...Ch. 11 - Prob. 11.1QPCh. 11 - Explain the term polarizability. What kind of...Ch. 11 - Prob. 11.3QPCh. 11 - Prob. 11.4QPCh. 11 - Prob. 11.5QPCh. 11 - Prob. 11.6QPCh. 11 - The compounds Br2 and ICl have the same number of...Ch. 11 - If you lived in Alaska, which of the following...Ch. 11 - The binary hydrogen compounds of the Group 4A...Ch. 11 - List the types of intermolecular forces that exist...Ch. 11 - Prob. 11.11QPCh. 11 - Prob. 11.12QPCh. 11 - Arrange the following in order of increasing...Ch. 11 - Diethyl ether has a boiling point of 34.5C, and...Ch. 11 - Which member of each of the following pairs of...Ch. 11 - Which substance in each of the following pairs...Ch. 11 - Prob. 11.17QPCh. 11 - What kind of attractive forces must be overcome in...Ch. 11 - The following compounds have the same molecular...Ch. 11 - Prob. 11.20QPCh. 11 - Explain why liquids, unlike gases, are virtually...Ch. 11 - What is surface tension? What is the relationship...Ch. 11 - Prob. 11.23QPCh. 11 - Prob. 11.24QPCh. 11 - A glass can be filled slightly above the rim with...Ch. 11 - Draw diagrams showing the capillary action of (a)...Ch. 11 - Prob. 11.27QPCh. 11 - Why does the viscosity of a liquid decrease with...Ch. 11 - Why is ice less dense than water?Ch. 11 - Outdoor water pipes have to be drained or...Ch. 11 - Predict which of the following liquids has greater...Ch. 11 - Predict the viscosity of ethylene glycol relative...Ch. 11 - Define the following terms: crystalline solid,...Ch. 11 - Describe the geometries of the following cubic...Ch. 11 - Classify the solid states in terms of crystal...Ch. 11 - The melting points of the oxides of the...Ch. 11 - What is the coordination number of each sphere in...Ch. 11 - Calculate the number of spheres that would be...Ch. 11 - Metallic iron crystallizes in a cubic lattice. The...Ch. 11 - Barium metal crystallizes in a body-centered cubic...Ch. 11 - Vanadium crystallizes in a body-centered cubic...Ch. 11 - Europium crystallizes in a body-centered cubic...Ch. 11 - Crystalline silicon has a cubic structure. The...Ch. 11 - A face-centered cubic cell contains 8 X atoms at...Ch. 11 - Define X-ray diffraction. What are the typical...Ch. 11 - Write the Bragg equation. Define every term and...Ch. 11 - When X rays of wavelength 0.090 nm are diffracted...Ch. 11 - The distance between layers in a NaCl crystal is...Ch. 11 - Describe and give examples of the following types...Ch. 11 - Prob. 11.50QPCh. 11 - A solid is hard, brittle, and electrically...Ch. 11 - A solid is soft and has a low melting point (below...Ch. 11 - Prob. 11.53QPCh. 11 - Which of the following are molecular solids and...Ch. 11 - Classify the solid state of the following...Ch. 11 - Prob. 11.56QPCh. 11 - Prob. 11.57QPCh. 11 - Define glass. What is the chief component of...Ch. 11 - What is a phase change? Name all possible changes...Ch. 11 - What is the equilibrium vapor pressure of a...Ch. 11 - Use any one of the phase changes to explain what...Ch. 11 - Define the following terms: (a) molar heat of...Ch. 11 - How is the molar heat of sublimation related to...Ch. 11 - What can we learn about the intermolecular forces...Ch. 11 - The greater the molar heat of vaporization of a...Ch. 11 - Define boiling point. How does the boiling point...Ch. 11 - As a liquid is heated at constant pressure, its...Ch. 11 - Prob. 11.68QPCh. 11 - Prob. 11.69QPCh. 11 - How do the boiling points and melting points of...Ch. 11 - Prob. 11.71QPCh. 11 - Wet clothes dry more quickly on a hot, dry day...Ch. 11 - Which of the following phase transitions gives off...Ch. 11 - A beaker of water is heated to boiling by a Bunsen...Ch. 11 - Calculate the amount of heat (in kJ) required to...Ch. 11 - Prob. 11.76QPCh. 11 - How is the rate of evaporation of a liquid...Ch. 11 - The molar heats of fusion and sublimation of...Ch. 11 - The following compounds, listed with their boiling...Ch. 11 - Prob. 11.80QPCh. 11 - A student hangs wet clothes outdoors on a winter...Ch. 11 - Steam at 100C causes more serious burns than water...Ch. 11 - Vapor pressure measurements at several different...Ch. 11 - Prob. 11.84QPCh. 11 - The vapor pressure of liquid X is lower than that...Ch. 11 - Explain why splashing a small amount of liquid...Ch. 11 - What is a phase diagram? What useful information...Ch. 11 - Explain how waters phase diagram differs from...Ch. 11 - The phase diagram of sulfur is shown. (a) How many...Ch. 11 - A length of wire is placed on top of a block of...Ch. 11 - Prob. 11.91QPCh. 11 - A phase diagram of water is shown at the end of...Ch. 11 - Name the kinds of attractive forces that must be...Ch. 11 - Prob. 11.94QPCh. 11 - Prob. 11.95QPCh. 11 - Prob. 11.96QPCh. 11 - Referring to Figure 11.41, determine the stable...Ch. 11 - Classify the unit cell of molecular iodine.Ch. 11 - A CO2 fire extinguisher is located on the outside...Ch. 11 - What is the vapor pressure of mercury at its...Ch. 11 - A flask of water is connected to a powerful vacuum...Ch. 11 - The liquid-vapor boundary line in the phase...Ch. 11 - Prob. 11.103QPCh. 11 - Prob. 11.104QPCh. 11 - In 2009, thousands of babies in China became ill...Ch. 11 - The vapor pressure of a liquid in a closed...Ch. 11 - A student is given four solid samples labeled W,...Ch. 11 - Prob. 11.108QPCh. 11 - Note the kettle of boiling water on a stove....Ch. 11 - The south pole of Mars is covered with dry ice,...Ch. 11 - The properties of gases, liquids, and solids...Ch. 11 - Select the substance in each pair that should have...Ch. 11 - Prob. 11.113QPCh. 11 - Under the same conditions of temperature and...Ch. 11 - The fluorides of the second-period elements and...Ch. 11 - The standard enthalpy of formation of gaseous...Ch. 11 - The following graph shows approximate plots of ln...Ch. 11 - Determine the final state and its temperature when...Ch. 11 - The distance between Li+ and Cl is 257 pm in solid...Ch. 11 - Heat of hydration, that is, the heat change that...Ch. 11 - Prob. 11.121QPCh. 11 - Calculate the H for the following processes at...Ch. 11 - Gaseous or highly volatile liquid anesthetics are...Ch. 11 - A beaker of water is placed in a closed container....Ch. 11 - The phase diagram of helium is shown. Helium is...Ch. 11 - Prob. 11.126QPCh. 11 - Ozone (O3) is a strong oxidizing agent that can...Ch. 11 - A sample of limestone (CaCO3) is heated in a...Ch. 11 - Silicon used in computer chips must have an...Ch. 11 - Carbon and silicon belong to Group 4A of the...Ch. 11 - Prob. 11.131QPCh. 11 - A 1.20-g sample of water is injected into an...Ch. 11 - What are the advantages of cooking the vegetable...Ch. 11 - A quantitative measure of how efficiently spheres...Ch. 11 - Provide an explanation for each of the following...Ch. 11 - Argon crystallizes in the face-centered cubic...Ch. 11 - A chemistry instructor performed the following...Ch. 11 - Given the phase diagram of carbon shown, answer...Ch. 11 - Swimming coaches sometimes suggest that a drop of...Ch. 11 - Prob. 11.140QPCh. 11 - Why do citrus growers spray their trees with water...Ch. 11 - What is the origin of dark spots on the inner...Ch. 11 - The compound dichlorodifluoromethane (CCl2F2) has...Ch. 11 - A student heated a beaker of cold water (on a...Ch. 11 - Sketch the cooling curves of water from about 110C...Ch. 11 - Iron crystallizes in a body-centered cubic...Ch. 11 - Prob. 11.147QPCh. 11 - Prob. 11.148QPCh. 11 - Prob. 11.149QPCh. 11 - A sample of water shows the following behavior as...Ch. 11 - Prob. 11.151QPCh. 11 - Assuming ideal behavior, calculate the density of...Ch. 11 - Both calcium and strontium crystallize in...Ch. 11 - Is the vapor pressure of a liquid more sensitive...Ch. 11 - Prob. 11.155IMECh. 11 - Without the aid of instruments, give two examples...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY