
(a)
Interpretation:
Whether the following molecule has a liquid crystalline phase or not should be determined.
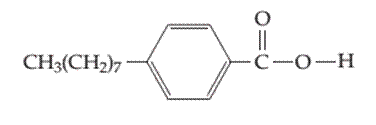
Concept introduction:
Liquid crystals are defined as a phase in which substance exhibits properties of both liquids and solids. Liquid crystal flow like a liquid but their arrangement of the molecule as well as intermolecular forces is like solid.
Liquid crystal molecules are made up of six-membered rings with on terminal polar group, a linkage group and a side chain of carbon atoms. Each carbon atom in liquid crystal molecules has trigonal planar geometry.
The molecules are rigid. The rigidity is increased due to the presence of double-bonded linkage groups such as
The terminal polar groups exhibit strong intermolecular forces such as strong dipole-dipole interaction or dipole−induced dipole interaction and hydrogen bond.
Types of liquid crystal are as follows:
- Nematic Liquid crystal.
- Smectic Liquid crystal.
1. Nematic Liquid crystal: The molecules in the nematic phase are in the same direction and can move around freely very much like that of liquid. In this, the axis is parallel but the ends are not aligned.
2. Smectic Liquid crystal: The molecules in the smectic phase are perpendicular to the plane and are aligned in layers. In these, the long axis is parallel and also their ends are aligned.
(b)
Interpretation:
Whether the following molecule has a liquid crystalline phase or not should be determined.
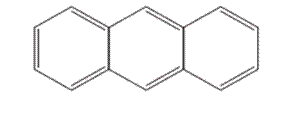
Concept introduction:
Liquid crystals are defined as a phase in which substance exhibits properties of both liquids and solids. Liquid crystal flow like a liquid but their arrangement of the molecule as well as intermolecular forces is like solid.
Liquid crystal molecules are made up of six-membered rings with on terminal polar group, a linkage group and a side chain of carbon atoms. Each carbon atom in liquid crystal molecules has trigonal planar geometry.
The molecules are rigid. The rigidity is increased due to the presence of double-bonded linkage groups such as
The terminal polar groups exhibit strong intermolecular forces such as strong dipole-dipole interaction or dipole−induced dipole interaction and hydrogen bond.
Types of liquid crystal are as follows:
- Nematic Liquid crystal.
- Smectic Liquid crystal.
1. Nematic Liquid crystal: The molecules in the nematic phase are in the same direction and can move around freely very much like that of liquid. In this, the axis is parallel but the ends are not aligned.
2. Smectic Liquid crystal: The molecules in the smectic phase are perpendicular to the plane and are aligned in layers. In these, the long axis is parallel and also their ends are aligned.
(c)
Interpretation:
Whether the following molecule has a liquid crystalline phase or not should be determined.
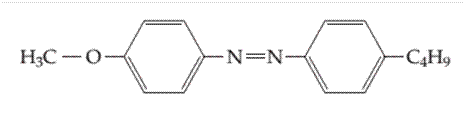
Concept introduction:
Liquid crystals are defined as a phase in which substance exhibits properties of both liquids and solids. Liquid crystal flow like a liquid but their arrangement of the molecule as well as intermolecular forces is like solid.
Liquid crystal molecules are made up of six-membered rings with on terminal polar group, a linkage group and a side chain of carbon atoms. Each carbon atom in liquid crystal molecules has trigonal planar geometry.
The molecules are rigid. The rigidity is increased due to the presence of double-bonded linkage groups such as
The terminal polar groups exhibit strong intermolecular forces such as strong dipole-dipole interaction or dipole−induced dipole interaction and hydrogen bond.
Types of liquid crystal are as follows:
- Nematic Liquid crystal.
- Smectic Liquid crystal.
1. Nematic Liquid crystal: The molecules in the nematic phase are in the same direction and can move around freely very much like that of liquid. In this, the axis is parallel but the ends are not aligned.
2. Smectic Liquid crystal: The molecules in the smectic phase are perpendicular to the plane and are aligned in layers. In these, the long axis is parallel and also their ends are aligned.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
- 32. The diagrams below show the band structure of an intrinsic semiconductor at absolute zero and room temperature. Room Temperature EF E OK Ep- a) In the space below, sketch a similar pair of diagrams for an n-type semiconductor. D) Give the definition and an example of (i) an intrinsic semiconductor and (ii) an n-type semiconductor.arrow_forward29. a) i Which energy diagram best represents the d-electrons in tetrahedral [Co(NH3)4]²+? b) ii c) iii d) iv 11 ་ ↑↓ ↑t t ↑↓ ↑↓ e) none of these ii In1 According to Slater's rules, what is the effective nuclear charge experienced by a 3d electron in 30. Ge? a) 32.00 b) 21.15 c) 16.05 d) 14.00 e) 10.85arrow_forwardRegarding Lowis structuros and geometrios, Draw Lewis structures for the following: SOF4, SO, ICI, XeO2F4, SeF and XeO3. For each one, indicate the observed molecular geometry it adopts.arrow_forward
- Explain the following statements with equations: - The fusion product of an organic compund with sodium metal is an alkaline solution - The test for elements should be done before the solubility tests. - Using less sodium than the organic compound in the ignition test might cause a problem to detect the presence of the nitrogen and sulfur - Formation of colored product when adding ferric acid chloride to phenol solutionarrow_forward31 Indicate the symbol, mass number and the atomic number of the missing product in each of the following nuclear reactions. a) 13/3 N 41 b) 11 Ca 20 c) 90 38 Sr → 133 C + ? + - 6 0 e →? 90 Y + ? 39 11 d) 22 Na → ? + + 1 B +1 β Toarrow_forwardPlease drawarrow_forward
- 9. compore the Following two Venctions IN termy Of Ronction Rate and explan in detail the reasoning that led to your conclusion +He p₁₂ 11- ㅐ 15 .. +He H #H H / H b. Compare the Following too reactions 14 terms of reaction Rate and explain in detail the reasoning that led to your conclusion Н d-C- tłu Na +2446 е -ll +2n "Harrow_forwarda. •Write all of the possible products For the Following ronction А ----- H - H H + H₂0 H+ Н b. in Rite the complete reaction Mechaniszn For the Formation of each product. ·C. Suggest what Reaction conditions could Result in each product being the major Product of the veaction:arrow_forwarda. Write the product For each of the Following reactions H 6-836-6 레 +H₂ N A H A-C-C=C-C-CH + 2 Na +2 NH3 - H H b. Write the reaction Mechanism For. reaction eacharrow_forward
- help draw the moleculearrow_forwardHow to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





