
Concept explainers
a)
Interpretation:
The structure of the
Concept introduction:
- The longest continuous ring of carbon atoms is identified. The prefix is added with ‘cyclo’.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
b)
Interpretation:
The structure of cis-2-butene has to be predicted.
Concept introduction:
IUPAC Nomenclature of
- The longest continuous chain of carbon atoms is identified.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
Geometric isomers of Alkenes:
Cis-isomer: When two particular atoms (identical group of atoms) are adjacent (on same side) to each other, the alkene is known as cis-isomer.
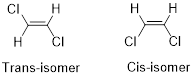
Trans-isomer: When two particular atoms (identical group of atoms) are opposite from each other, the alkene is known as trans-isomer.
c)
Interpretation:
The structure of 2-hexanol has to be predicted.
Concept introduction:
IUPAC Nomenclature of alkanes:
- The longest continuous chain of carbon atoms is identified.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
d)
Interpretation:
The structure of 1,4-dibromobenzene has to be predicted.
Concept introduction:
IUPAC Nomenclature of
- The parent compound of aromatic compound is Benzene.
- The substituent groups attached to the parent is identified. A substituent group contains group of atoms attached to the carbon atom of the aromatic ring.
- While numbering the parent, the location of the second group relative to the first substituent uses prefixes like o-ortho (1,2-), m-meta (1,3-), and p-para (1,4) in disubstituted aromatic ring.
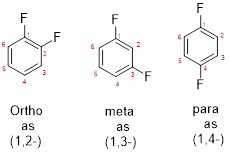
e)
Interpretation:
The structure of 2-butyne has to be predicted.
Concept introduction:
IUPAC Nomenclature of
- The longest continuous chain of carbon atoms is identified. The suffix is added replaced with ‘yne’.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Connect 1 Semester Access Card for General Chemistry: The Essential Concepts
- What are the missing intermediates 1, 2, and 3? Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediates and how they occur.arrow_forwardWhat is the reactant that makes the following product of the reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardDraw the products formed when each ester is hydrolyzed with water and sulfuric acid.arrow_forward
- Draw the complete structural formula from each condensed structure. include all hydrogen atoms.arrow_forwardDraw the complete structural formula from each condensed structure. Include all hydrogen atoms.arrow_forwardIndicate how H2O2 intervenes in the synthesis of K4[Co2(C2O4)4(OH)2]. Write the reactions.arrow_forward
- Explain how, based on physical gas adsorption isotherms, we can determine whether multi-walled C nanotubes are open at their ends. Explain this.arrow_forwardcan somone answer pleasearrow_forwardConstruct a molecular orbital energy-level diagram for BeH2. Sketch the MO pictures (schematic representation) for the HOMO and LUMO of BeH2 [Orbital Potential Energies, H (1s): -13.6 eV; Be (2s): -9.3 eV, Be (2p): -6.0 eV]arrow_forward
- Indicate the isomers of the A(H2O)6Cl3 complex. State the type of isomerism they exhibit and explain it briefly.arrow_forwardState the formula of the compound potassium μ-dihydroxydicobaltate (III) tetraoxalate.arrow_forwardConsider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





